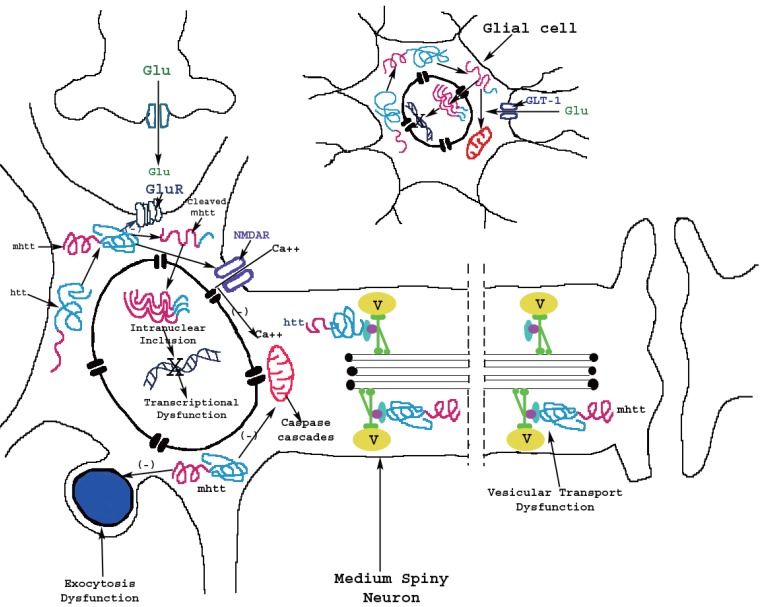
Figure 1.
Model for mechanism of actions of mutant huntingtin protein (mhtt) in medium spiny neuron and glial cell in Huntington’s disease. The huntingtin protein is transformed to mhtt through unknown mechanism, genetically or environmentally, in both neuron and glial cell. There are several theories suggesting different actions of mhtt: 1) mhtt protein may interact with metabotropic (GluR) or ionotropic glutamatergic (NMDA) receptors and alters their function, 2) the mhtt protein may bind to cellular transport components and induce vesicular transport (V) or exocytosis dysfunction. Moreover, mhtt protein might be proteolytically cleaved in amino-terminal fragments which form β-sheet structures. In cytoplasm, the cleaved mhtt may interact with mitochondria and alters their function. In addition, the cleaved mhtt can enter the nucleus and forms intranuclear aggregates or intranuclear inclusions, which induce transcriptional dysfunction. Both mutant full-length and cleaved forms of htt may form soluble monomers, oligomers or large insoluble aggregates. Similar mechanism of the actions of cleaved mhtt is suggested to occur in glial cells. The cleaved mhtt may alter glutamatergic system such as glutamate transporter 1 (GLT-1) and consequently alters the uptake of glutamate.