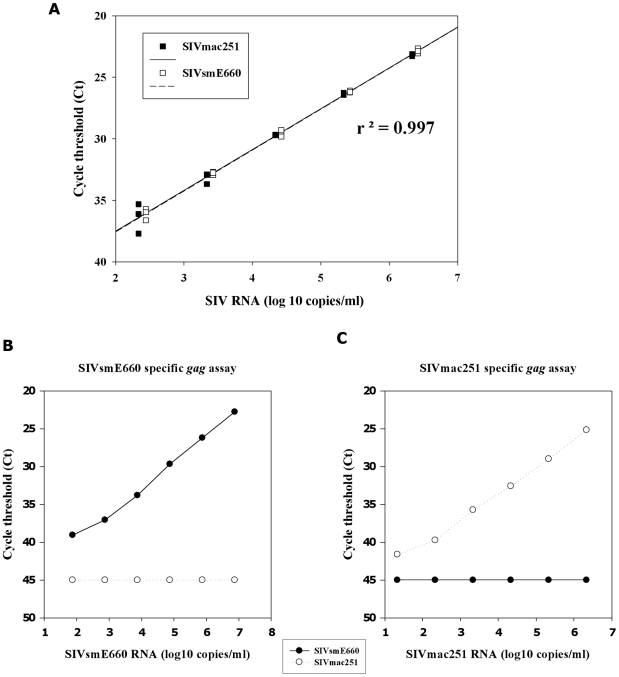
Figure 7. Type common and type specific PCR analysis of SIVmac251 and SIVsmE660.
Panel A shows equivalent amplification of SIVmac251 and SIVsmE660 with R/U5 primers targeting the 5′ region of genomic SIV RNA. Comparable regression curves were obtained with a high titre SIVmac251 reference panel [65] and a SIVsmE660 plasma pool derived from day 10 and 14 bleeds of titration macaques B1–B4. Subsequent cross-titration experiments conducted with the heterologous gag-based SIVsmE660 and SIVmacC8 plasma viral RNA quantification assays compared threshold detection levels with viral RNA in-put copy number. Assay specificity was demonstrated with the same pooled SIVsmE660 plasma from control MCMs diluted in negative plasma and a SIVmac251/L28 plasma reference panel [65] for SIVmac251 sequences. Respective assays demonstrated specific amplification of the SIVsmE660 (challenge, panel B) and SIVmac251 (vaccine, panel C) viruses by gag-specific RT-PCR across a 6 log10 dynamic range. Intra-assay variation between replicates of the SIVsmE660-specific assay was 0.09 SD with a minimum amplification efficiency of 94.9%. Replicates included at least three runs with a coefficient of variation of <3%. No cross-reactivity with the heterologous virus was detected above a sensitivity of detection limit of 50 SIV RNA copies/ml.