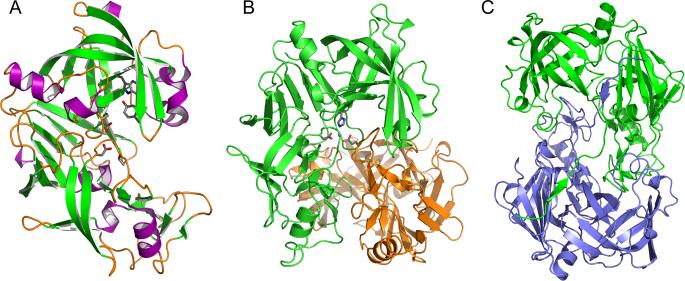
Figure 2.
Structures of plasmepsins. (A) Three-dimensional structure of apo-PMII. The secondary structural elements are shown in different colors (magenta for helices, green for strands, and orange for loops and irregular structural elements). Selected residues important for the catalytic mechanism are shown in stick representation. (B) The structure of the HAP dimer (green and orange) in its apo form, with the side chains in one of the active sites shown in stick representation. A Zn2+ ion bound in the active site is shown as a sphere. (C) A unique domain swapped dimer of the HAP-KNI-10395 complex viewed down the 2-fold axis. One protomer is shown in green and the other one in blue.