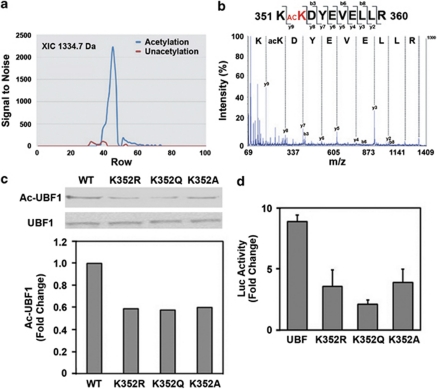
Figure 5.
UBF1 is acetylated at K352 residue by CBP, and UBF1 acetylation site mutants (K352A, K352Q, and K352R) decreases the transcriptional activity of rDNA. (a) Comparison of LC-MALDI-MS/MS extracted ion chromatogram between in vitro-acetylated UBF1–HMG3 domain peptide (acetylation) and untreated control UBF1–HMG3 domain peptide (unacetylation). The extracted ion chromatogram (XIC) of the precursor ion at m/z 1334.7 Da was plotted as signal to nose (vertical) versus spot number (horizontal). (b) MALDI-TOF/TOF MS/MS spectrum of acetylated peptide KacKDYEVELLR (1334.7 m/z) from human UBF1 HMG3 domain (accession number: P17480). The chart represents m/z (horizontal) versus intensity (vertical). (c) Mutation of UBF1 at K352 to A, Q, and R reduced its acetylation level in neuronal cells. pCMV–Flag–UBF1 s (WT, K352A, K352Q, and K352R) were transiently transfected, immunoprecipitaed with anti-Flag antibody and blotted with anti-Ac-Lys antibody. (d) UBF1 acetylation site mutants (K352A, K352Q, and K352R) decreases the transcriptional activity of rDNA in response to CBP