Abstract
Schistosomes are the causative agents of schistosomiasis, a neglected tropical disease affecting hundreds of millions worldwide and a major global health burden. Current control of schistosomiasis depends largely on a single drug, praziquantel (PZQ). One potential physiological target for new antischistosomal drugs is the parasite's excretory system, which removes wastes and xenobiotics. Multidrug resistance (MDR) transporters that are members of the ATP-binding cassette (ABC) superfamily of proteins are ATP-dependent efflux pumps involved in removal of toxins and xenobiotics from cells. They mediate the phenomenon of multidrug resistance, in which cells resistant to one drug show cross-resistance to a broad range of other agents, and are also associated with reduced drug susceptibility in parasitic helminths. In this review, we survey the different types of ABC transporter genes present within the schistosome genome, and examine recent evidence indicating that at least some of these transporters may play a role in fine-tuning susceptibility of schistosomes to PZQ. Disruption of their function may therefore provide a strategy for enhancing drug action or overcoming or attenuating drug resistance. Furthermore, dissection of the roles these transporters may play in normal schistosome physiology could potentially lead to identification of highly “druggable” targets for new antischistosomals.
1. Introduction
The most recent assessments suggest that schistosomiasis affects over 400 million people worldwide, with as many as 280,000 deaths per year in Africa alone (King, 2010, van der Werf et al., 2003). The global health burden of schistosomiasis has been estimated at 9–36 million disability-adjusted life years (DALYs), a value similar to that of malaria or tuberculosis (Hotez and Fenwick, 2009, King, 2010). Chemotherapy continues to be the primary intervention for schistosomiasis, and is the main force in its control. Praziquantel (PZQ), the current drug of choice against schistosomiasis (Caffrey, 2007, Doenhoff et al., 2009, Hagan et al., 2004), is effective against all human schistosome species, has relatively mild side effects, and is able to cure schistosomiasis in a single dose (or 2–3 divided doses). The value of PZQ has been demonstrated repeatedly in large-scale schistosomiasis control efforts in a variety of countries (Toure et al., 2008, Vennervald et al., 2005). Indeed, because of these advantages, as well as steadily reduced costs, PZQ now serves as the only commercially available antischistosomal treatment in most parts of the world (Fenwick et al., 2003, Hagan et al., 2004). However, such reliance on a single drug represents a precarious situation (Caffrey, 2007), particularly in light of reports of schistosome isolates with reduced susceptibility to PZQ (Day and Botros, 2006, Doenhoff and Pica-Mattoccia, 2006, Melman et al., 2009). Schistosomes also show major stage-specific differences in PZQ susceptibility; immature worms are highly refractory to PZQ, which means that treatment is not fully effective until approximately 6 weeks post-infection (Aragon et al., 2009, Pica-Mattoccia and Cioli, 2004, Sabah et al., 1986, Xiao et al., 1985). Furthermore, the mode of PZQ action remains incompletely defined (Doenhoff et al., 2008, Greenberg, 2005, Redman et al., 1996); this lack of a definite mechanism makes the possibility of emerging PZQ resistance especially daunting. One strategy that has been proposed to overcome drug resistance, as well as to enhance drug efficacy, is to potentiate current anthelmintics by including additional agents targeted against different, but perhaps interacting, sites of action, or against cellular components that regulate rates of drug uptake, metabolism, or efflux. Multidrug resistance transporters have been suggested as particularly attractive targets of this type (Lespine et al., 2008, Liang and Aszalos, 2006).
2. Multidrug resistance transporters
Similar to other organisms, schistosomes must take up nutrients and excrete toxic metabolic wastes, and they must do so within the presumably hostile environment of the host circulatory system. Efflux of metabolic toxins and xenobiotics is mediated by several types of transporters, a subset of which are members of the ATP binding cassette (ABC) superfamily of transporters identified initially by their role in multidrug resistance (MDR). MDR was first described in mammalian tumor cells which were selected for resistance to a single drug, but which demonstrated unexpected cross-resistance to several structurally unrelated compounds. The phenomenon is linked to amplification and overexpression of MDR transporters, resulting in increased drug efflux. MDR transporters include P-glycoprotein (Pgp), which was the first of these transporters identified, multidrug resistance-associated proteins (MRPs), breast cancer resistance protein (BCRP), and others (Ambudkar et al., 2003, Gimenez-Bonafe et al., 2008). ABC transporter genes have been subsequently divided into subfamilies based on gene structure, order of domains, and sequence similarity (Dean et al., 2001, Leonard et al., 2003). In mammals, there are seven ABC gene subfamilies, labeled ABCA through ABCG. Each of these families comprises from one (ABCE) to several (ABCA, ABCB, ABCC) representatives. For example, the gene for Pgp, which is also known as MDR1, is ABCB1, the gene for MRP1 is ABCC1, and the gene for BCRP is ABCG2. The human genome is estimated to contain almost 50 different ABC transporter genes (Dean et al., 2001).
The function of MDR transporters in normal cellular physiology is to remove or exclude xenobiotics and metabolic toxins from cells, as well as to transport signaling molecules. They also play essential roles in a wide variety of physiological processes (Johnstone et al., 2000, Mizutani et al., 2008) including regulation of apoptosis (Johnstone et al., 2000), immune responses (van de Ven et al., 2009), and possibly tumor promotion (Fletcher et al., 2010). MDR transporters are likely to be important components of normal parasite physiology as well, and there is increasing evidence that they additionally affect drug susceptibility in helminths (Ardelli et al., 2005, Ardelli et al., 2006, Bartley et al., 2009, Blackhall et al., 1998, Blackhall et al., 2008, Kerboeuf et al., 2003, Kumkate et al., 2008, Messerli et al., 2009, Sangster et al., 1999, Xu et al., 1998). The potential role of these transporters in helminth and other parasite drug resistance has recently been reviewed (James et al., 2009, James et al., 2009, Jones and George, 2005, Kerboeuf et al., 2003, Lespine et al., 2008).
3. ABC transporters in schistosomes
Several years ago, two ABC transporter cDNAs were cloned from S. mansoni. One of them, SMDR2, codes for a protein that resembles Pgp, with two putative ATP-binding domains and 12 predicted transmembrane segments; the other, SMDR1, is a half-transporter, with only a single predicted ATP-binding domain (Bosch et al., 1994). With the publication of the S. mansoni genome (Berriman et al., 2009), several other S. mansoni genes predicted to encode ABC transporters are now available (see Table 1). Some of the more potentially interesting representatives include orthologs of MRP1/ABCC1 (SmMRP1); MRP4/ABCC4 (Smp_083750, Smp_167610); BCRP/ABCG2 (Smp_126450, Smp_137890); the lipid transporter ABCA4 (Smp_056290); ABCB7 (Smp_087930), a mitochondrial transporter involved in biogenesis of iron-sulfur clusters (Zutz et al., 2009); and ABCB6 (Smp_134890), which is likely involved in heme biosynthesis (Zutz et al., 2009). There also appear to be at least four predicted Pgp-like transporters in addition to SMDR2 (Table 1). Many of these gene products are almost certain to play critical roles in schistosome physiology and parasite/host interactions.
Table 1.
List of ABC transporter genes found in the S. mansoni genome.
| Human gene family | Accession Number |
|---|---|
| ABCA* | Smp_176450 |
| ABCA3 | Smp_165800 |
| ABCA4 | Smp_056290 |
| ABCB1 (SMDR2, Pgp) | L26287, Smp_035720, Smp_055780 |
| ABCB1 (other Pgp) | Smp_089200 |
| Smp_110180 | |
| Smp_137080 | |
| Smp_170820 | |
| ABCB6 | Smp_134890 |
| ABCB7 | Smp_087930 |
| ABCB8 (SMDR1) | L26286, Smp_063000 |
| ABCC1 (SmMRP1) | GU967672, Smp_171740 |
| ABCC1 (other MRP1) | Smp_129820 |
| Smp_189630 | |
| ABCC4 | Smp_083750 |
| Smp_167610 | |
| ABCC10 (MRP7) | Smp_147250 |
| ABCE1 | Smp_124460 |
| ABCF1 | Smp_166040 |
| ABCF2 | Smp_040540 |
| ABCF3 | Smp_049490 |
| ABCG1 | Smp_181150 |
| ABCG2 (BCRP) | Smp_126450 |
| Smp_137890 | |
| ABCG4 | Smp_094330 |
BLAST/FASTA unable to resolve subclass
4. S. mansoni ABC transporters and drug susceptibility
In the original publication of Bosch et al. (1994), SMDR2 RNA was shown to be expressed at higher levels in adult female schistosomes than in males. We have confirmed that pattern for SMDR2 RNA levels, but surprisingly find higher Pgp-immunoreactive protein in males compared with females. In contrast to SMDR2, RNA for SmMRP1, the S. mansoni MRP1-like sequence, is expressed at higher levels in males than in females, and male and female adults show differential anti-MRP1 localization (Kasinathan et al., 2010a). Thus, in adult male parasites, anti-MRP1 immunoreactivity localizes most prominently to the testes and the gut epithelium, while in females, anti-MRP1 immunoreactivity is found near the excretory pore and in subtegumental regions.
Bosch et al. (1994) found no indication of higher expression in two hycanthone/oxamniquine-resistant isolates, suggesting that increases in Pgp expression were not associated with drug resistance, at least for those isolates. Since that time, PZQ has almost entirely supplanted other antischistosomal drugs. Hence, we decided to investigate the relationship between PZQ and this class of transporters. We were encouraged in part by reports showing that fluorescent substrates for Pgp and MRP localized to the excretory system of schistosomes (Sato et al., 2002, Sato et al., 2004), that PZQ disrupted that localization (Kusel et al., 2006, Oliveira et al., 2006), and that PZQ had relatively potent inhibitory activity against mammalian Pgp (Hayeshi, et al., 2006)..
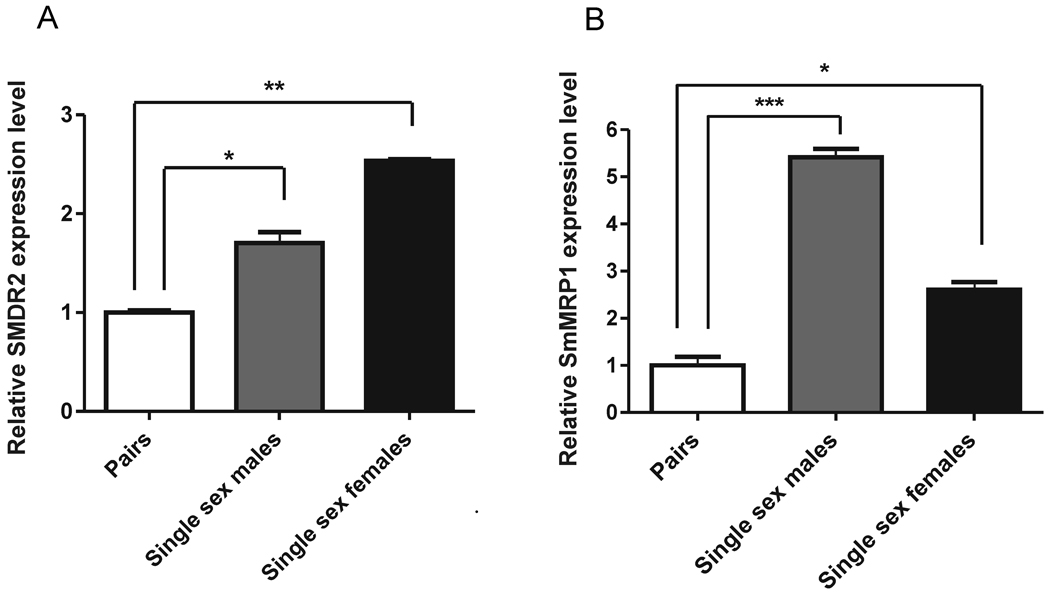
We first examined whether exposure of adult schistosomes to sub-lethal concentrations of PZQ changes expression levels of S. mansoni Pgp (SMDR2) or MRP1 (SmMRP1). We found that both SMDR2 and SmMRP1 are transiently upregulated in response to 100–300 nM PZQ (Kasinathan et al., 2010, Messerli et al., 2009). Appearance of prominent anti-Pgp immunoreactivity within and surrounding the gut occurs in males exposed to PZQ. These findings might hint at a link for development of PZQ resistance. Indeed, in every case we have examined, reduced sensitivity to PZQ correlates with increased expression of one or both of these MDR transporters. Thus, EE2, an Egyptian S. mansoni isolate with reduced (2–4-fold) PZQ susceptibility, shows dramatically higher levels of SMDR2 (but not SmMRP1) RNA (approximately 10-fold) and protein (Kasinathan et al., 2010a, Messerli et al., 2009); PZQ-refractory juvenile worms express significantly higher (approximately 2.5-fold) levels of both SMDR2 and SmMRP1 RNAs (Kasinathan et al., 2010a); and worms from single-sex infections, which are less susceptible to PZQ (Pica-Mattocia and Cioli, 2004), also express higher levels of both SMDR2 and SmMRP1 (Fig. 1). Interestingly, Couto et al. (2010) have shown that exposure to PZQ of an S. mansoni isolate with reduced PZQ susceptibility retains normal labeling of the parasite excretory system by the fluorescent Pgp substrate resorufin, in contrast to the disruption of labeling seen in PZQ-susceptible worms
Fig. 1. Worms from single-sex infections express higher levels of both SmMRP1 and SMDR2 RNA than do paired worms.
qRT-PCR analysis (SYBR) of RNA extracted from S. mansoni 6–7 weeks post-infection. Parasites were perfused from mice infected with males and females (white bar), single-sex males (grey bar), or single-sex females (black bar). Primers were directed against SMDR2 or SmMRP1, and the reference gene was 18S RNA. (A) Relative SMDR2 RNA expression (n= 3) (B) Relative SmMRP1 RNA expression (n= 3–4). *, **, *** indicate P<0.05, P<0.01, and P<0.001, respectively, ANOVA.
5. SMDR2 pharmacology
The observations described above suggested a possible association between PZQ action and susceptibility and schistosome multidrug transporters. However, the mechanism that might link the PZQ and these transporters remained unclear. In order to better understand a possible connection, we expressed SMDR2 in mammalian Chinese Hamster Ovary cells and analyzed its pharmacological properties (Kasinathan et al., 2010b). We found that rhodamine transport via SMDR2 is, as might be expected, blocked by inhibitors of mammalian Pgp such as verapamil and nifedipine. As with mammalian Pgp, SMDR2 is also inhibited with relatively high potency by PZQ (IC50 = 17.4 µM). This IC50 value is far higher than the sub-micromolar concentrations of PZQ capable of eliciting the initial contractile response of S. mansoni adults in vitro. However, it falls within the range of PZQ concentrations reported to be required to kill S. mansoni adults in vitro (or prevent their recovery from effects of PZQ exposure); these measurements have been made by several groups, each using different methodologies and assays for lethality, with reported EC50 values ranging from approximately 3 µM to >100 µM (Aragon et al., 2009, Pica-Mattoccia and Cioli, 2004, Xiao et al., 1985). Estimates of PZQ concentrations found in vivo, following administration to the mammalian host, range from a peak plasma concentration of 0.8 µM in humans (following a 20 mg/kg oral dose), to 68 µM in the portal blood of rats (following a 300 mg/kg dose) (reviewed by Andrews, 1985). Active PZQ appears to be eliminated less rapidly when given to schistosome-infected mice compared with uninfected mice, and has been measured at 10 µM in peripheral plasma of S. mansoni-infected mice 1 h following administration of a 100 mg/kg PZQ dose (Andrews, 1985).
We additionally found that, unlike mammalian Pgp, for which PZQ is not a substrate (Hayeshi et al., 2006), SMDR2 does appear capable of transporting PZQ, or at least a BODIPY-conjugated version of PZQ (Kasinathan et al., 2010b). These results may begin to provide a connection between the upregulation of schistosome MDR transporters and exposure to PZQ, as well as a model for MDR-mediated reduction in PZQ susceptibility. The molecular target for PZQ remains undefined, though our evidence suggests involvement of a novel calcium channel β subunit subtype found only in platyhelminths (Greenberg, 2005). Calcium channel β subunits are cytoplasmic proteins associated with the membrane-bound, pore-forming α1 subunit of the channel. Other postulated PZQ targets include both membrane and intracellular proteins. However, as Aragon et al.(2009) point out, how the initial effects of PZQ on parasite muscle contraction and paralysis relate to ultimate worm death remains an open question. SMDR2 (and perhaps other MDR transporters) may be involved in excreting PZQ from schistosome cells, thus reducing its intracellular concentration. In fact, SMDR2 may be precluding PZQ from even crossing the cell membrane, transporting it out of the lipid bilayer before it has an opportunity to enter the cell. Increased levels of SMDR2 could therefore prevent interaction of PZQ with its target(s) more efficiently, thus attenuating the lethal effects of the drug.
6. Conclusions
These findings indicate that schistosome MDR transporters may be important for fine-tuning schistosome drug susceptibility. On the other hand, they provide only limited information about the possible role of these transporters in normal schistosome physiology. One way to begin to answer this question is to examine the phenotypes that result following pharmacological or genetic disruption of these transporters. We are currently testing the effects of knockdown of SMDR2 and SmMRP1 RNAs by RNAi and pharmacological inhibition of these transporters with a variety of MDR inhibitors. Our preliminary results indicate that either treatment disrupts parasite egg production. Such results would suggest that, in addition to their possible role in modulating drug action and susceptibility, these types of transporters are playing either a direct critical role in schistosome reproduction, or possibly a role in some other parasite function that indirectly impacts egg production. Further experimentation should resolve this issue. However, it is nonetheless clear that from the totality of our (and others') results that schistosome (and possibly other helminth) MDR transporters are likely to be eminently useful as “druggable” targets on their own, or perhaps as enhancers of current anthelmintics. Of course, agents that selectively target parasite transporters would be preferred, and our results indicating differences in the ability of SMDR2 and mammalian Pgp to transport PZQ suggest that selective targeting may be feasible. Likely, only very short-term administration of agents targeting these transporters would be necessary, thereby overcoming some of the challenges of using these drugs in long-term regimens, as would be required for cancer treatment (Coley, 2010, Shukla et al., 2008).
Acknowledgments
This work was supported by NIH grant R01 AI073660 and in part by NIH grant R21 AI082390. The single-sex infected mice were obtained from the Schistosomiasis Resource Center (Rockville, MD) through NIH-NIAID Contract N01 AI30026. We thank William Morgan for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambudkar SV, Kimchi-Sarfaty C, Sauna ZE, Gottesman MM. P-glycoprotein: from genomics to mechanism. Oncogene. 2003;22:7468–7485. doi: 10.1038/sj.onc.1206948. [DOI] [PubMed] [Google Scholar]
- Andrews P. Praziquantel: mechanisms of anti-schistosomal activity. Pharmac Ther. 1985;29:129–156. doi: 10.1016/0163-7258(85)90020-8. [DOI] [PubMed] [Google Scholar]
- Andrews P. A summary of the efficacy of praziquantel against schistosomes in animal experiments and notes on its mode of action. Arzneimittel-Forschung. 1981;31:538–541. [PubMed] [Google Scholar]
- Aragon AD, Imani RA, Blackburn VR, Cupit PM, Melman SD, Goronga T, Webb T, Loker ES, Cunningham C. Towards an understanding of the mechanism of action of praziquantel. Mol Biochem Parasit. 2009;164:57–65. doi: 10.1016/j.molbiopara.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardelli BF, Guerriero SB, Prichard RK. Genomic organization and effects of ivermectin selection on Onchocerca volvulus P-glycoprotein. Mol Biochem Parasit. 2005;143:58–66. doi: 10.1016/j.molbiopara.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Ardelli BF, Guerriero SB, Prichard RK. Ivermectin imposes selection pressure on P-glycoprotein from Onchocerca volvulus: linkage disequilibrium and genotype diversity. Parasitology. 2006;132:375–386. doi: 10.1017/S0031182005008991. [DOI] [PubMed] [Google Scholar]
- Bartley DJ, McAllister H, Bartley Y, Dupuy J, Menez C, Alvinerie M, Jackson F, Lespine A. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology. 2009;136:1081–1088. doi: 10.1017/S0031182009990345. [DOI] [PubMed] [Google Scholar]
- Berriman M, Haas BJ, LoVerde PT, Wilson RA, Dillon GP, Cerqueira GC, Mashiyama ST, Al-Lazikani B, Andrade LF, Ashton PD, Aslett MA, Bartholomeu DC, Blandin G, Caffrey CR, Coghlan A, Coulson R, Day TA, Delcher A, DeMarco R, Djikeng A, Eyre T, Gamble JA, Ghedin E, Gu Y, Hertz-Fowler C, Hirai H, Hirai Y, Houston R, Ivens A, Johnston DA, Lacerda D, Macedo CD, McVeigh P, Ning Z, Oliveira G, Overington JP, Parkhill J, Pertea M, Pierce RJ, Protasio AV, Quail MA, Rajandream MA, Rogers J, Sajid M, Salzberg SL, Stanke M, Tivey AR, White O, Williams DL, Wortman J, Wu W, Zamanian M, Zerlotini A, Fraser-Liggett CM, Barrell BG, El-Sayed NM. The genome of the blood fluke Schistosoma mansoni. Nature. 2009;460:352–358. doi: 10.1038/nature08160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhall WJ, Liu HY, Xu M, Prichard RK, Beech RN. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol Biochem Parasit. 1998;95:193–201. doi: 10.1016/s0166-6851(98)00087-5. [DOI] [PubMed] [Google Scholar]
- Blackhall WJ, Prichard RK, Beech RN. P-glycoprotein selection in strains of Haemonchus contortus resistant to benzimidazoles. Vet Parasitol. 2008;152:101–107. doi: 10.1016/j.vetpar.2007.12.001. [DOI] [PubMed] [Google Scholar]
- Bosch IB, Wang ZX, Tao LF, Shoemaker CB. Two Schistosoma mansoni cDNAs encoding ATP-binding cassette (ABC) family proteins. Mol Biochem Parasit. 1994;65:351–356. doi: 10.1016/0166-6851(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Caffrey CR. Chemotherapy of schistosomiasis: present and future. Curr Opin Chem Biol. 2007;11:433–439. doi: 10.1016/j.cbpa.2007.05.031. [DOI] [PubMed] [Google Scholar]
- Coley HM. Overcoming multidrug resistance in cancer: clinical studies of p-glycoprotein inhibitors. Methods Mol Biol. 2010;596:341–358. doi: 10.1007/978-1-60761-416-6_15. [DOI] [PubMed] [Google Scholar]
- Day TA, Botros S. Drug resistance in schistosomes. In: Maule A, Marks NJ, editors. Parasitic Flatworms: Molecular Biology, Biochemistry, Immunology and Physiology. Oxfordshire, UK: CAB International; 2006. pp. 256–268. [Google Scholar]
- Dean M, Rzhetsky A, Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11:1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- Doenhoff MJ, Cioli D, Utzinger J. Praziquantel: mechanisms of action, resistance and new derivatives for schistosomiasis. Curr Opin Infecti Dis. 2008;21:659–667. doi: 10.1097/QCO.0b013e328318978f. [DOI] [PubMed] [Google Scholar]
- Doenhoff MJ, Hagan P, Cioli D, Southgate V, Pica-Mattoccia L, Botros S, Coles G, Tchuem Tchuente LA, Mbaye A, Engels D. Praziquantel: its use in control of schistosomiasis in sub-Saharan Africa and current research needs. Parasitology. 2009:1–11. doi: 10.1017/S0031182009000493. [DOI] [PubMed] [Google Scholar]
- Doenhoff MJ, Pica-Mattoccia L. Praziquantel for the treatment of schistosomiasis: its use for control in areas with endemic disease and prospects for drug resistance. Expert Rev Anti Infect Ther. 2006;4:199–210. doi: 10.1586/14787210.4.2.199. [DOI] [PubMed] [Google Scholar]
- Fenwick A, Savioli L, Engels D, Robert Bergquist N, Todd MH. Drugs for the control of parasitic diseases: current status and development in schistosomiasis. Trends Parasitol. 2003;19:509–515. doi: 10.1016/j.pt.2003.09.005. [DOI] [PubMed] [Google Scholar]
- Fletcher JI, Haber M, Henderson MJ, Norris MD. ABC transporters in cancer: more than just drug efflux pumps. Nature Rev.Cancer. 2010;10:147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- Gimenez-Bonafe P, Guillen Canovas A, Ambrosio S, Tortosa A, Perez-Tomas R. In: Drugs modulating MDR. Colabufo NA, editor. Kerala, India: Research Signpost; 2008. pp. 63–99. [Google Scholar]
- Greenberg RM. Are Ca2+ channels targets of praziquantel action? Int J Parasitol. 2005;35:1–9. doi: 10.1016/j.ijpara.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Hagan P, Appleton CC, Coles GC, Kusel JR, Tchuem-Tchuente LA. Schistosomiasis control: keep taking the tablets. Trends Parasitol. 2004;20:92–97. doi: 10.1016/j.pt.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Hayeshi R, Masimirembwa C, Mukanganyama S, Ungell AL. The potential inhibitory effect of antiparasitic drugs and natural products on P-glycoprotein mediated efflux. Eur J Pharm Sci. 2006;29:70–81. doi: 10.1016/j.ejps.2006.05.009. [DOI] [PubMed] [Google Scholar]
- Hotez PJ, Fenwick A. Schistosomiasis in Africa: an emerging tragedy in our new global health decade. PLoS Neglect Trop D. 2009;3:e485. doi: 10.1371/journal.pntd.0000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CE, Hudson AL, Davey MW. Drug resistance mechanisms in helminths: is it survival of the fittest? Trends Parasitol. 2009;25:328–335. doi: 10.1016/j.pt.2009.04.004. [DOI] [PubMed] [Google Scholar]
- James CE, Hudson AL, Davey MW. An update on P-glycoprotein and drug resistance in Schistosoma mansoni. Trends Parasitol. 2009;25:538–539. doi: 10.1016/j.pt.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Smyth MJ. Multiple physiological functions for multidrug transporter P-glycoprotein? Trends Biochem Sci. 2000;25:1–6. doi: 10.1016/s0968-0004(99)01493-0. [DOI] [PubMed] [Google Scholar]
- Johnstone RW, Ruefli AA, Tainton KM, Smyth MJ. A role for P-glycoprotein in regulating cell death. Leukemia Lymphoma. 2000;38:1–11. doi: 10.3109/10428190009060314. [DOI] [PubMed] [Google Scholar]
- Jones PM, George AM. Multidrug resistance in parasites: ABC transporters, P-glycoproteins and molecular modelling. Int J Parasitol. 2005;35:555–566. doi: 10.1016/j.ijpara.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Kasinathan RS, Goronga T, Messerli SM, Webb TR, Greenberg RM. Modulation of a Schistosoma mansoni multidrug transporter by the antischistosomal drug praziquantel. FASEB J. 2010b;24:128–135. doi: 10.1096/fj.09-137091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasinathan RS, Morgan WM, Greenberg RM. Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel. Mol Biochem Parasit. 2010a;173:25–31. doi: 10.1016/j.molbiopara.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerboeuf D, Blackhall W, Kaminsky R, von Samson-Himmelstjerna G. P-glycoprotein in helminths: function and perspectives for anthelmintic treatment and reversal of resistance. Int J Antimicrob Ag. 2003;22:332–346. doi: 10.1016/s0924-8579(03)00221-8. [DOI] [PubMed] [Google Scholar]
- King CH. Schistosomiasis: challenges and opportunities, Institute of Medicine, Board on Global Health, Forum on Microbial Threats. Institute of Medicine. 2010 http://iom.edu/~/media/Files/Activity%20Files/PublicHealth/MicrobialThreats/2010-SEP-21/King%20CH.pdf. [Google Scholar]
- Kumkate S, Chunchob S, Janvilisri T. Expression of ATP-binding cassette multidrug transporters in the giant liver fluke Fasciola gigantica and their possible involvement in the transport of bile salts and anthelmintics. Mol Cell Biochem. 2008;317:77–84. doi: 10.1007/s11010-008-9833-2. [DOI] [PubMed] [Google Scholar]
- Kusel JR, Oliveira FA, Todd M, Ronketti F, Lima SF, Mattos AC, Reis KT, Coelho PM, Thornhill JA, Ribeiro F. The effects of drugs, ions, and poly-l-lysine on the excretory system of Schistosoma mansoni. Mem I Oswaldo Cruz. 2006;101(Suppl 1):293–298. doi: 10.1590/s0074-02762006000900046. [DOI] [PubMed] [Google Scholar]
- Leonard GD, Fojo T, Bates SE. The role of ABC transporters in clinical practice. The Oncologist. 2003;8:411–424. doi: 10.1634/theoncologist.8-5-411. [DOI] [PubMed] [Google Scholar]
- Lespine A, Alvinerie M, Vercruysse J, Prichard RK, Geldhof P. ABC transporter modulation: a strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 2008;24:293–298. doi: 10.1016/j.pt.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Liang XJ, Aszalos A. Multidrug transporters as drug targets. Curr Drug Targets. 2006;7:911–921. doi: 10.2174/138945006778019264. [DOI] [PubMed] [Google Scholar]
- Melman SD, Steinauer ML, Cunningham C, Kubatko LS, Mwangi IN, Wynn NB, Mutuku MW, Karanja DM, Colley DG, Black CL, Secor WE, Mkoji GM, Loker ES. Reduced susceptibility to praziquantel among naturally occurring Kenyan isolates of Schistosoma mansoni. PLoS Neglect Trop D. 2009;3:e504. doi: 10.1371/journal.pntd.0000504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerli SM, Kasinathan RS, Morgan W, Spranger S, Greenberg RM. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol Biochem Parasit. 2009;167:54–59. doi: 10.1016/j.molbiopara.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizutani T, Masuda M, Nakai E, Furumiya K, Togawa H, Nakamura Y, Kawai Y, Nakahira K, Shinkai S, Takahashi K. Genuine functions of P-glycoprotein (ABCB1) Curr Drug Metab. 2008;9:167–174. doi: 10.2174/138920008783571756. [DOI] [PubMed] [Google Scholar]
- Oliveira FA, Kusel JR, Ribeiro F, Coelho PM. Responses of the surface membrane and excretory system of Schistosoma mansoni to damage and to treatment with praziquantel and other biomolecules. Parasitology. 2006;132:321–330. doi: 10.1017/S0031182005009169. [DOI] [PubMed] [Google Scholar]
- Pica-Mattoccia L, Cioli D. Sex- and stage-related sensitivity of Schistosoma mansoni to in vivo and in vitro praziquantel treatment. Int J Parasitol. 2004;34:527–533. doi: 10.1016/j.ijpara.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Redman CA, Robertson A, Fallon PG, Modha J, Kusel JR, Doenhoff MJ, Martin RJ. Praziquantel: an urgent and exciting challenge. Parasitol Today. 1996;12:14–20. doi: 10.1016/0169-4758(96)80640-5. [DOI] [PubMed] [Google Scholar]
- Sabah AA, Fletcher C, Webbe G, Doenhoff MJ. Schistosoma mansoni: chemotherapy of infections of different ages. Exp Parasitol. 1986;61:294–303. doi: 10.1016/0014-4894(86)90184-0. [DOI] [PubMed] [Google Scholar]
- Sangster NC, Bannan SC, Weiss AS, Nulf SC, Klein RD, Geary TG. Haemonchus contortus: sequence heterogeneity of internucleotide binding domains from P-glycoproteins. Exp Parasitol. 1999;91:250–257. doi: 10.1006/expr.1998.4373. [DOI] [PubMed] [Google Scholar]
- Sato H, Kusel JR, Thornhill J. Functional visualization of the excretory system of adult Schistosoma mansoni by the fluorescent marker resorufin. Parasitology. 2002;125:527–535. doi: 10.1017/s0031182002002536. [DOI] [PubMed] [Google Scholar]
- Sato H, Kusel JR, Thornhill J. Excretion of fluorescent substrates of mammalian multidrug resistance-associated protein (MRP) in the Schistosoma mansoni excretory system. Parasitology. 2004;128:43–52. doi: 10.1017/s0031182003004177. [DOI] [PubMed] [Google Scholar]
- Shukla S, Wu CP, Ambudkar SV. Development of inhibitors of ATP-binding cassette drug transporters: present status and challenges. Expert Opin Drug Metab Toxicol. 2008;4:205–223. doi: 10.1517/17425255.4.2.205. [DOI] [PubMed] [Google Scholar]
- Toure S, Zhang Y, Bosque-Oliva E, Ky C, Ouedraogo A, Koukounari A, Gabrielli AF, Bertrand S, Webster JP, Fenwick A. Two-year impact of single praziquantel treatment on infection in the national control programme on schistosomiasis in Burkina Faso. B World Health Organ. 2008;86:780–787. doi: 10.2471/BLT.07.048694. A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Ven R, Scheffer GL, Scheper RJ, de Gruijl TD. The ABC of dendritic cell development and function. Trends Immunol. 2009;30:421–429. doi: 10.1016/j.it.2009.06.004. [DOI] [PubMed] [Google Scholar]
- van der Werf MJ, de Vlas SJ, Brooker S, Looman CW, Nagelkerke NJ, Habbema JD, Engels D. Quantification of clinical morbidity associated with schistosome infection in sub-Saharan Africa. Acta Trop. 2003;86:125–139. doi: 10.1016/s0001-706x(03)00029-9. [DOI] [PubMed] [Google Scholar]
- Vennervald BJ, Booth M, Butterworth AE, Kariuki HC, Kadzo H, Ireri E, Amaganga C, Kimani G, Kenty L, Mwatha J, Ouma JH, Dunne DW. Regression of hepatosplenomegaly in Kenyan school-aged children after praziquantel treatment and three years of greatly reduced exposure to Schistosoma mansoni. T Roy Soc Trop Med H. 2005;99:150–160. doi: 10.1016/j.trstmh.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Xiao SH, Catto BA, Webster LT., Jr Effects of praziquantel on different developmental stages of Schistosoma mansoni in vitro and in vivo. J Infect Dis. 1985;151:1130–1137. doi: 10.1093/infdis/151.6.1130. [DOI] [PubMed] [Google Scholar]
- Xu M, Molento M, Blackhall W, Ribeiro P, Beech R, Prichard R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol Biochem Parasit. 1998;91:327–335. doi: 10.1016/s0166-6851(97)00215-6. [DOI] [PubMed] [Google Scholar]
- Zutz A, Gompf S, Schagger H, Tampe R. Mitochondrial ABC proteins in health and disease. Biochim Biophys Acta. 2009;1787:681–690. doi: 10.1016/j.bbabio.2009.02.009. [DOI] [PubMed] [Google Scholar]