Abstract
TGF-β1 contributes to chronic kidney disease, at least in part, via Smad3. TGF-β1 is induced in the kidney following acute ischemia, and there is increasing evidence that TGF-β1 may protect against acute kidney injury. As there is a paucity of information regarding the functional significance of Smad3 in acute kidney injury, the present study explored this issue in a murine model of ischemic acute kidney injury in Smad3+/+ and Smad3−/− mice. We demonstrate that, at 24 h after ischemia, Smad3 is significantly induced in Smad3+/+ mice, whereas Smad3−/− mice fail to express this protein in the kidney in either the sham or postischemic groups. Compared with Smad3+/+ mice, and 24 h following ischemia, Smad3−/− mice exhibited greater preservation of renal function as measured by blood urea nitrogen (BUN) and serum creatinine; less histological injury assessed by both semiquantitative and qualitative analyses; markedly suppressed renal expression of IL-6 and endothelin-1 mRNA (but comparable expression of MCP-1, TNF-α, and heme oxygenase-1 mRNA); and no increase in plasma IL-6 levels, the latter increasing approximately sixfold in postischemic Smad3+/+ mice. We conclude that genetic deficiency of Smad3 confers structural and functional protection against acute ischemic injury to the kidney. We speculate that these effects may be mediated through suppression of IL-6 production. Finally, we suggest that upregulation of Smad3 after an ischemic insult may contribute to the increased risk for chronic kidney disease that occurs after acute renal ischemia.
Keywords: IL-6, TGF-β1, heme oxygenase-1, chronic kidney disease
transforming growth factor-β1 (TGF-β1) is a pleiotropic cytokine that affects many of the major pathobiological processes that contribute to tissue injury (4, 7, 8, 18, 30, 33, 40, 42, 49). Such effects of TGF-β1 are commonly cell specific and context dependent (18, 33); for example, depending upon the experimental conditions, TGF-β1 may promote processes that are either antiapoptotic or proapoptotic and may exert actions that are either proinflammatory or anti-inflammatory. Such diverse and, at times, seemingly contradictory effects of TGF-β1 probably reflect the myriad signaling systems recruited by TGF-β1, and these include 1) the Smad family of proteins, 2) the MAPK system, 3) phosphatidylinositol 3-kinase signaling, 4) small GTPases, 5) mammalian target of rapamycin, and 6) ILK (4, 7, 8, 18, 30, 33, 40, 42, 49). However, whatever the divergent, cell-specific effects of TGF-β1 on biological processes, one effect of TGF-β1 is firmly and consistently established irrespective of the involved tissues: the capacity of TGF-β1 to promote matrix expansion and fibrogenesis, processes in which the signaling intermediate, Smad3, is a fundamental participant. Indeed, TGF-β1 is uniformly regarded as a key contributor to the progression of chronic kidney disease, a contribution that reflects, at least in part, the signaling effects of Smad3 (4, 7, 8, 30, 40, 42, 49, 51).
The pathobiological significance of TGF-β1 in acute kidney injury is increasingly of interest (2, 3, 14, 15, 26–28, 45). Early and marked upregulation of TGF-β1 and its receptors occurs following acute renal ischemia (2, 3, 14, 45), and, as shown by our prior studies, TGF-β1 is markedly induced in a sustained fashion following heme protein-induced renal injury (36). Studies in the rat model of acute renal ischemia demonstrate that antagonism of the actions of TGF-β1 inhibits the synthesis of extracellular matrix proteins, but fails to influence the course of renal function following such ischemic injury (14, 45). However, in other models of acute ischemic injury, a cytoprotective role for TGF-β1 has been demonstrated. In a murine model of acute renal ischemia, a deficiency in TGF-β1 exacerbates renal dysfunction and worsens renal histological injury (15), and in in vitro models inhibition of TGF-β1 exacerbates oxidant-induced necrosis of proximal tubular epithelial cells (27). Acquired resistance to renal ischemic injury in certain settings is also TGF-β1 dependent. For example, prior exposure to the anesthetic agent sevoflurane protects against renal damage following acute ischemic insults, and such protection by sevoflurane is impaired either by the administration of a neutralizing TGF-β1 antibody, or in TGF-β1+/− mice (26). In studies in vitro, a neutralizing TGF-β1 antibody vitiates the cytoprotection conferred by sevoflurane against hydrogen peroxide-induced injury to proximal tubular epithelial cells (27).
In light of evidence that TGF-β1 is protective in acute kidney injury, and the fundamental role of Smad3 in promoting the injurious effects of TGF-β1, at least in chronic kidney disease, the present study was undertaken to examine the role of Smad3 in ischemic acute kidney injury.
MATERIALS AND METHODS
Murine Smad3−/− model.
Smad3+/+ and Smad3−/− mice employed for the present studies were generated from colonies of mice established from breeder stock obtained from the Jackson Laboratory (129-Smad3tm1Par/J, stock no. 003451, Bar Harbor, ME), and maintained by mating Smad3+/− males with Smad3+/− females. Offspring were genotyped at the time of weaning using PCR to amplify the wild-type and mutant alleles of genomic DNA from tail samples. For studies of Smad3−/− and Smad3+/+ mice, age-matched male mice from 10 to 24 wk were employed. Additionally, in other studies 14-wk-old male C57BL6J mice (Jackson Laboratory) were employed. All experiments were performed in accordance with the guidelines of the National Institutes of Health and approved by the Institutional Animal Care and Use Committee of the Mayo Clinic.
Murine model of renal ischemia-reperfusion.
This model of acute renal ischemia was induced in mice as described in detail by our prior studies (20, 21, 37, 39, 47). Briefly, after mice were anesthetized with pentobarbital (50 mg/kg ip), the renal pedicles were gently dissected and bilateral renal ischemia was induced with nontraumatic clamps (RS5426, Micro Aneurysm clip, straight, 10 mm, 125-g pressure; Roboz Surgical Instruments, Rockville, MD) through a midline abdominal incision. Two protocols of renal ischemia consisting of either 22.5 or 25 min were separately employed in Smad3+/+ and Smad3−/− mice so as to determine the consistency of the observed effect of the deficiency of Smad3 on different durations of renal ischemia. Sham procedures of the duration of the 25-min ischemia procedure included the abdominal incision but excluded renal pedicle dissection and clamping. For these studies, renal function was assessed by the measurement of serum creatinine and blood urea nitrogen (BUN) levels at 24 h after ischemia using a Creatinine Analyzer 2 and a BUN Analyzer 2 (Beckman Instruments, Fullerton, CA).
mRNA expression by quantitative real-time RT-PCR.
For analysis of gene expression, total RNA was extracted from snap-frozen mouse renal tissues using the TRIzol method (Invitrogen, Carlsbad, CA) and subsequently further purified with an RNeasy Mini kit (Qiagen, Valencia, CA), according to each manufacturer's protocol and as described in detail in our prior studies (39, 48). Three hundred nanograms of purified total RNA were used in 30-μl reverse transcription reactions (Transcriptor First Strand cDNA Synthesis kit, Roche Applied Science, Indianapolis, IN) employing random hexamers. The resulting cDNA was used in quantitative real-time PCR analysis as in our earlier study (39). Reactions were performed on an ABI Prism 7900HT (Applied Biosystems, Foster City, CA) using TaqMan Mastermix reagent (part no. 4324020, Applied Biosystems). Probes and primers obtained as assay sets (TaqMan Gene Expression Assays, Applied Biosystems) were employed in these reactions according to the manufacturer's protocol. Parameters for quantitative PCR were as follows: 10 min at 95°C, followed by 40 cycles of amplification for 15 s at 95°C, and 1 min at 60°C. Expression of 18S rRNA was used for standardization of the expression of each target gene.
Western blot analysis.
Western blot analysis was performed as described in our previous studies (39, 48). Briefly, proteins (150 μg) were separated on 10% Tris-HCl gels (Bio-Rad) and transferred to polyvinylidene fluoride membranes. Primary antibodies for Smad3 (catalog no. 9523, Cell Signaling Technology, Danvers, MA) and β-actin (catalog no. 612657, BD Biosciences, San Jose, CA) were used in overnight incubations at 4°C. Horseradish peroxidase-conjugated secondary antibodies were then used, and bands were visualized using an enhanced chemiluminescence method.
Serum IL-6 quantitation by ELISA.
Serum concentrations of IL-6 protein were measured using a commercially available ELISA set (OptEIA, catalog no. 555240, BD Biosciences) according to the manufacturer's assay instructions.
Histological analysis.
Histological analysis was performed on 5-μm hematoxylin- and eosin-stained sections prepared from formalin-fixed, paraffin-embedded renal tissues from Smad3+/+ and Smad3−/− mice 24 h after bilateral renal ischemia or sham operation. Blinded semiquantitative evaluation of necrosis was performed by assessing the percentage of tubules demonstrating any evidence of epithelial cell necrosis, and the degree of extension of necrosis into the cortex, determined by the mean percentage of the distance from the corticomedullary junction to the surface of the kidney in which necrosis was present.
Statistics.
Data are expressed as means ± SE. Data for Smad3−/− and Smad3+/+ mice for a given condition were compared using Student's t-test for parametric data and the Mann-Whitney test for nonparametric data. Results were considered significant at P < 0.05.
RESULTS
Effect of renal ischemia on Smad3 expression.
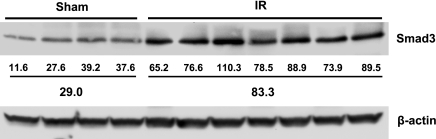
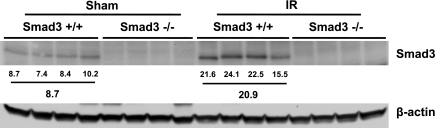
At 3 h, Smad3 expression by Western blot analysis was unaltered in response to renal ischemia compared with sham ischemia in wild-type mice (data not shown). However, at a later time point, 24 h, there was a marked and significantly increased expression (∼3-fold) of Smad3 in Smad3+/+ mice subjected to renal ischemia (Fig. 1). Western blot analysis also confirmed that Smad3−/− mice failed to express Smad3 in the kidney after either sham ischemia or renal ischemia, thereby confirming the deficiency of this protein in this mutant strain (Fig. 2).
Fig. 1.
Western blot analysis of Smad3 expression in kidneys of Smad3+/+ mice 24 h after bilateral renal ischemia (IR) for 25 min or sham surgery (Sham). Each lane represents protein extracted from a single kidney of an individual mouse. Equivalency of protein loading was assessed by immunoblotting for β-actin, and individual and mean standardized densitometric values are provided below the Western blot analysis. Smad3 expression was significantly higher in mice subjected to IR compared with mice subjected to Sham (P < 0.05).
Fig. 2.
Western blot analysis of Smad3 expression in kidneys of Smad3+/+ and Smad3−/− mice 24 h after IR for 25 min or Sham. Each lane represents protein extracted from a single kidney of an individual mouse. Equivalency of protein loading was assessed by immunoblotting for β-actin, and individual and mean standardized densitometric values are provided below the Western blot analysis. No expression of Smad3 occurred in Smad3−/− mice.
Effect of Smad3 deficiency on renal function.
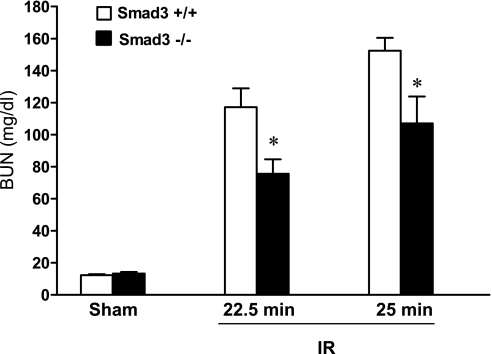
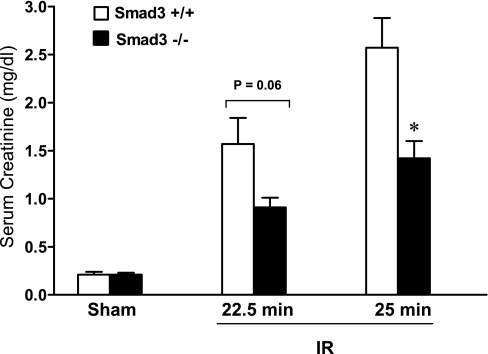
Renal functional studies were undertaken in two separate protocols, employing different durations of ischemia that lead to duration-dependent, renal dysfunction in wild-type mice. As demonstrated, Smad3−/− mice exhibited substantial preservation in renal function in response to renal ischemia as reflected by BUN (Fig. 3) and serum creatinine (Fig. 4) 24 h after renal ischemia.
Fig. 3.
Serum blood urea nitrogen (BUN) levels 24 h after IR for either 22.5 or 25 min in duration and after Sham in Smad3+/+ and Smad3−/− mice; n = 7–8/group. *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to respective IR duration.
Fig. 4.
Serum creatinine levels 24 h after IR for either 22.5 or 25 min in duration and after Sham in Smad3+/+ and Smad3−/− mice; n = 7–8/group. *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to respective IR duration.
Effect of Smad3 deficiency on renal histological injury.
Along with this preservation of renal function in Smad3−/− mice following renal ischemia, there was substantial reduction in renal histological injury as reflected by less acute tubular necrosis, and less intratubular sloughing and cast formation in Smad3−/− mice following renal ischemia (Fig. 5). Semiquantitative analyses confirmed such qualitative analyses: scores for the severity of acute tubular necrosis and the extent to which tubular necrosis extended from the corticomedullary junction into the cortex were both significantly lower in Smad3−/− mice following renal ischemia either 22.5 or 25 min in duration (Fig. 6).
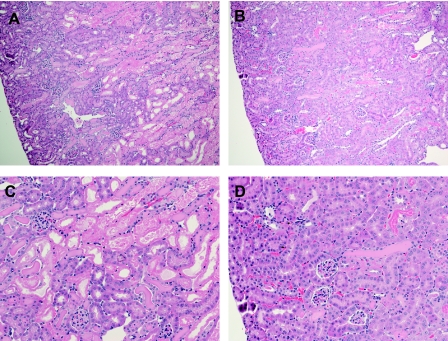
Fig. 5.
Histological examination of the kidney after IR in Smad3+/+ and Smad3−/− mice. Lower power (×100, A and B) and higher power (×200, C and D) views of the kidney in Smad3+/+ (A and C) and Smad3−/− (B and D) mice 24 h after IR for 25 min are shown. Smad3−/− mice exhibited less renal injury compared with Smad3+/+ mice in response to this duration of ischemia. No histological injury was observed in the kidneys of Smad3+/+ and Smad3−/− mice subjected to Sham (not shown). Tissue sections were stained with hematoxylin and eosin.
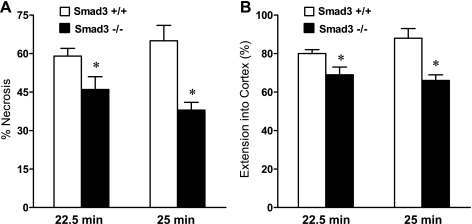
Fig. 6.
Semiquantitative assessment of necrosis in the kidney 24 h after IR for either 22.5 or 25 min in duration in Smad3+/+ and Smad3−/− mice. Shown are blinded analyses of the percentage of tubules exhibiting evidence of epithelial cell necrosis (A) and extension of cortical necrosis (B), the latter determined by the mean percentage of the distance from the corticomedullary junction to the surface of the kidney in which necrosis was present. No necrosis was observed in the kidneys of Smad3+/+ and Smad3−/− mice subjected to Sham (data not shown). *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to respective IR duration.
Effect of Smad3 deficiency on renal gene expression.
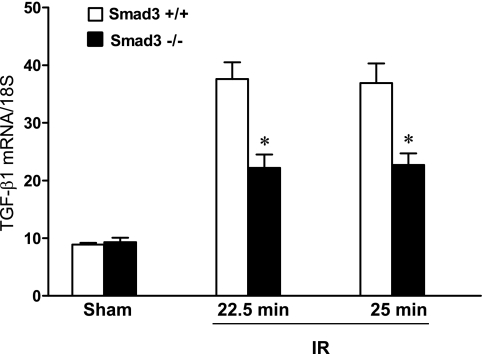
In an attempt to determine the basis for this effect of Smad3 deficiency, we assessed the expression of cytokines that contribute to ischemic acute kidney injury. As previously described, expression of IL-6 mRNA is markedly increased after renal ischemia, and this effect was greatly attenuated in Smad3−/− mice compared with Smad3+/+ mice following renal ischemia (Fig. 7). While not as striking, expression of endothelin-1 was also significantly reduced in Smad3−/− mice compared with Smad3+/+ mice following ischemia (Fig. 8). Interestingly, a similar pattern was found with TGF-β1 (Fig. 9). Other cytokines that are well established as contributors to ischemic acute kidney injury, specifically, MCP-1 and TNF-α, were not significantly altered by Smad3 deficiency (Table 1). In addition to pathways that promote renal injury, we also examined a pathway that can protect against renal injury, namely, heme oxygenase (HO)-1 (19, 35): HO-1 mRNA expression was comparably induced, thus demonstrating that this was unlikely to be a mechanism contributing to the protective effects of Smad3 deficiency (Table 1).
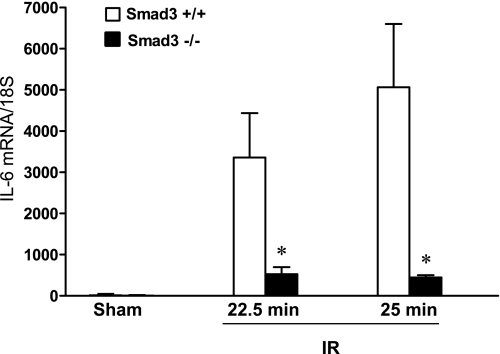
Fig. 7.
Renal expression of IL-6 mRNA 24 h after IR for either 22.5 or 25 min in duration and after Sham in Smad3+/+ and Smad3−/− mice. IL-6 mRNA expression was determined by quantitative real-time RT-PCR and standardized for 18S rRNA; n = 7–8/group. *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to respective IR duration.
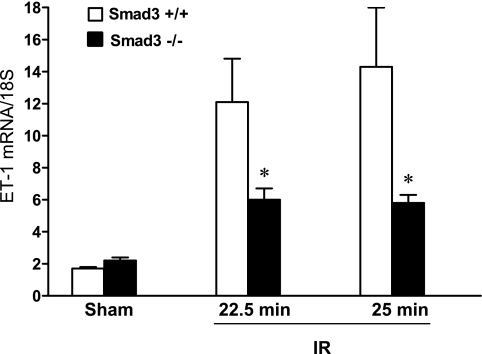
Fig. 8.
Renal expression of ET-1 mRNA 24 h after IR for either 22.5 or 25 min in duration and after Sham in Smad3+/+ and Smad3−/− mice. ET-1 mRNA expression was determined by quantitative real-time RT-PCR and standardized for 18S rRNA;. n = 7–8/group. *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to respective IR duration.
Fig. 9.
Renal expression of TGF-β1 mRNA 24 h after IR for either 22.5 or 25 min in duration and after Sham in Smad3+/+ and Smad3−/− mice. TGF-β1 mRNA expression was determined by quantitative real-time RT-PCR and standardized for 18S rRNA; n = 7–8/group. *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to respective IR duration.
Table 1.
mRNA expression assessed by quantitative real-time RT-PCR
| Ischemia-Reperfusion |
||||||
|---|---|---|---|---|---|---|
| Sham |
22.5 min |
25 min |
||||
| Smad3+/+ | Smad3−/− | Smad3+/+ | Smad3−/− | Smad3+/+ | Smad3−/− | |
| HO-1 | 7.9 ± 1.1 | 11.9 ± 3.7 | 57.1 ± 11.9 | 35.1 ± 6.5 | 62.8 ± 8.3 | 47.9 ± 11.0 |
| MCP-1 | 5.9 ± 1.1 | 5.8 ± 0.7 | 62.5 ± 5.5 | 49.6 ± 6.7 | 55.6 ± 7.1 | 53.0 ± 6.1 |
| TNF-α | 5.6 ± 1.1 | 8.1 ± 1.8 | 63.0 ± 6.0 | 53.4 ± 6.7 | 51.8 ± 4.9 | 44.5 ± 6.9 |
Values are means ± SE and are the results of relative quantification performed against a standard curve constructed for each mRNA target, normalized for expression of 18S rRNA and expressed in arbitrary units; n = 7–8 for all groups. HO-1, heme oxygenase-1.
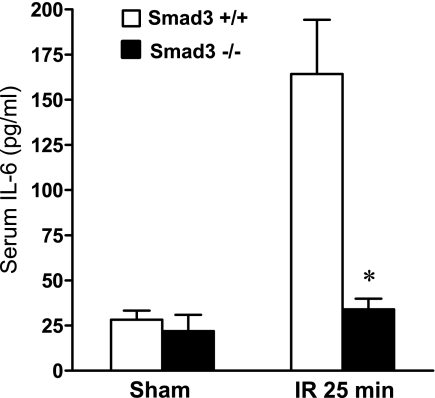
Effect of Smad3 deficiency on plasma IL-6 levels.
We also measured plasma levels of IL-6, since systemic levels of cytokines are a determinant of the outcome from acute kidney injury. Following ischemia, IL-6 was markedly increased at 24 h in Smad3+/+ mice, and such elevation in IL-6 after ischemia did not occur in Smad3−/− mice (Fig. 10).
Fig. 10.
Serum concentrations of IL-6 protein 24 h after IR for 25 min in duration and after Sham in Smad3+/+ and Smad3−/− mice. IL-6 serum levels were determined by ELISA; n = 7–8/group. *P < 0.05, Smad3−/− vs. Smad3+/+ mice subjected to IR.
DISCUSSION
Our data, in aggregate, demonstrate that Smad3 is induced in the kidney following acute renal ischemia and that genetic deficiency of Smad3 leads to greater preservation of renal function, less histological injury, reduced renal expression of specific cytokines, and less systemic inflammation following acute renal ischemia. Based on these findings, we conclude that induction of Smad3 is a maladaptive response that contributes to the functional and structural damage attendant upon acute renal ischemia.
In an attempt to uncover mechanisms that may underlie the reduced ischemic injury incurred in Smad3−/− mice, we evaluated the expression of a number of cytokines that contribute to acute kidney injury following ischemia. The most dramatic effect was noted in the renal expression of IL-6, the latter markedly lower in Smad3−/− mice compared with Smad3+/+ mice following ischemia. These findings are of interest in that multiple lines of evidence attest to the injurious effects of IL-6 following an ischemic insult. For example, the administration of a neutralizing IL-6 antibody leads to greater preservation of renal function and less histological injury following acute renal ischemic injury (22, 38); IL-6−/− mice evince less renal dysfunction and histological injury in response to an acute ischemic insult (22, 38); HO-1−/− mice, compared with HO-1+/+ mice, exhibit an exaggerated induction of IL-6, increased renal dysfunction, and increased mortality following renal ischemia (47), while the administration of a neutralizing IL-6 antibody attenuates such renal dysfunction and mortality observed in HO-1−/− mice following ischemia (47). Clinical observations support the pathogenetic significance of increased IL-6 production as increased urinary excretion of IL-6 is a predictor for human acute kidney injury (9, 25). Our finding that IL-6 mRNA was markedly induced in the ischemic kidney, and that such expression of IL-6 was drastically reduced in the ischemic kidney in Smad3−/− mice, raises the possibility that the protection conferred by Smad3 deficiency may reflect reduced production of IL-6. In this regard, observations in other cell types demonstrate that the induction of IL-6 by TGF-β1 critically requires Smad3 (1, 13).
Renal ischemia provokes a systemic inflammatory response, and among the recognized and prominent cytokines so produced is IL-6. Such a systemic response is implicated in the adverse distant effects of renal ischemia such as lung injury (23). Clinical observations in acutely ill patients demonstrate that plasma IL-6 levels predict ventilator dependency, severity of acute kidney injury, and mortality that occur in this patient population (6, 29, 44). Our data demonstrate that the marked elevation in plasma IL-6 levels observed following ischemia in wild-type mice did not occur in Smad3−/− mice. Thus the suppressive effect of the deficiency of Smad3 on IL-6 occurs not only regionally in the kidney but also in the systemic circulation.
It is possible that the protective effect of Smad3 deficiency we observed may reflect decreased production of other cytokines that are recognized as contributors to acute ischemic injury; in this regard, substantial evidence indicates that renal injury following acute ischemia can reflect increased renal expression of endothelin-1, MCP-1, and TNF-α (10–12, 17, 24, 31, 34, 50). Our data demonstrate that Smad3−/− mice compared with Smad3+/+ mice, in response to ischemia, had a blunted expression of ET-1 mRNA, and thus such reduced expression in ET-1 may be relevant to the reduced renal injury in Smad3−/− mice observed after ischemia. These findings are consistent with observations that cellular induction of ET-1 by TGF-β1 requires Smad3 (5, 41).
The finding that MCP-1 and TNF-α expression was comparably increased in the kidney in Smad3+/+ and Smad3−/− mice is of interest for at least two reasons. First, it is unlikely that the adverse effects of Smad3 in acute renal ischemia are mediated through these cytokines; second, the lack of alteration in expression of these cytokines indicates that the reduction in IL-6 and ET-1 was not a nonspecific, generalized reductive effect on cytokine expression attendant upon the deficiency of Smad3.
We observed that renal induction of TGF-β1 mRNA was substantially reduced in Smad3-deficient mice following ischemia. To the best of our knowledge, we are unaware of studies examining tissue expression of TGF-β1 in Smad3-deficient mice following an imposed stress in vivo. While the basis for this reduced TGF-β1 mRNA expression in stressed Smad3-deficient mice is uncertain, we speculate that a positive feedback loop exists between TGF-β1 and Smad3 in the ischemic kidney: in response to ischemia, TGF-β1 induces Smad3, and induced Smad3, in turn, fosters TGF-β1 expression.
Our finding that Smad3, a signaling molecule downstream of TGF-β1, contributes to ischemic acute kidney injury merits discussion within the context of TGF-β1 as a cytokine that protects against ischemic acute kidney injury (15, 26–28). TGF-β1 is pleiotropic in its actions and engages diverse signaling molecules. Even within the Smad family, constituent members exert divergent effects. For example, quite recently it has been shown that Smad2 offsets the fibrogenic actions of TGF-β1/Smad3 signaling (32). TGF-β1 also engages Smad7, which is a robust inducer of HO-1(16), a cytoprotective molecule that reduces acute kidney injury incurred by ischemia, heme proteins, nephrotoxins, and endotoxins (19, 35, 43). Presumably, the salutary effects of TGF-β1 in ischemic renal injury reflect the preferential elicitation or the preponderating effects of cytoprotective rather than injurious signaling pathways. Our findings delineate that the effect of Smad3-dependent pathways is that of promoting, rather than protecting against, acute ischemic renal injury.
A novel and important investigative line has recently demonstrated that prior exposure to the volatile anesthetic sevoflurane confers protection against acute ischemic renal injury (26–28). These studies demonstrate involvement of TGF-β1 and Smad3 in mediating the protective effects of sevoflurane. Included in these studies are control groups in which Smad3−/− mice were subjected to renal ischemia under pentobarbital sodium anesthesia; however, renal dysfunction, as measured by serum creatinine, under these conditions was comparable in Smad3+/+ and Smad3−/− mice (26). In this regard, we wish to point out the following considerations. First, the model of ischemia used in these studies (unilateral ischemia of 30 min and contralateral nephrectomy) differs from ours (bilateral ischemia of a duration of 22.5 and 25 min), and this difference in the model of renal ischemia as well as experimental conditions may be relevant. Second, the protective effect we observed at a given duration of ischemia (22.5 min) was reproduced at another duration (25 min). Third, we employed two different markers of glomerular filtration, namely, BUN and serum creatinine. Fourth, in one of our protocols of renal ischemia (22.5), the degree of renal dysfunction in Smad3+/+ mice at 24 h (serum creatinine ∼1.5 mg/dl) was comparable to that observed in Smad3+/+ mice 24 h after ischemia reported in prior studies.
In summary, to the best of our knowledge, we provide the first demonstration that induction of Smad3 occurs following acute ischemic insults, and such induction of Smad3 represents a maladaptive response that promotes acute ischemic injury. We suggest that the adverse effects of Smad3 reflect the upregulation of IL-6, which is known to occur in the ischemic kidney. The adverse effect of Smad3 in acute kidney injury described in the current study is notable in that other established mediators of chronic kidney disease [for example, TGF-β1 and hypoxia-inducible factor-1α (46)] are protective rather than contributory to acute ischemic injury. We also speculate that such upregulation of Smad3 may exert adverse long-term consequences. In our prior studies we described a sequential, triphasic response in the kidney subjected to repeated exposure to an acute insult: initial sensitivity, acquired resistance, and then chronic inflammation (36). In light of the established role of Smad3 in chronic inflammation and matrix expansion, we speculate that upregulation of Smad3 after acute renal ischemia may be germane to the risk of chronic kidney disease that ensues after acute ischemia.
GRANTS
These studies were supported by National Institutes of Health Grants DK47060, HL55552, and HL85307 (K. A. Nath and J. P. Grande).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
ACKNOWLEDGMENTS
We gratefully acknowledge the secretarial expertise of Tammy Engel.
REFERENCES
- 1. Aoki H, Ohnishi H, Hama K, Shinozaki S, Kita H, Yamamoto H, Osawa H, Sato K, Tamada K, Sugano K. Existence of autocrine loop between interleukin-6 and transforming growth factor-beta1 in activated rat pancreatic stellate cells. J Cell Biochem 99: 221–228, 2006 [DOI] [PubMed] [Google Scholar]
- 2. Basile DP, Martin DR, Hammerman MR. Extracellular matrix-related genes in kidney after ischemic injury: potential role for TGF-β in repair. Am J Physiol Renal Physiol 275: F894–F903, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Basile DP, Rovak JM, Martin DR, Hammerman MR. Increased transforming growth factor-β1 expression in regenerating rat renal tubules following ischemic injury. Am J Physiol Renal Fluid Electrolyte Physiol 270: F500–F509, 1996 [DOI] [PubMed] [Google Scholar]
- 4. Bottinger EP. TGF-beta in renal injury and disease. Semin Nephrol 27: 309–320, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Castanares C, Redondo-Horcajo M, Magan-Marchal N, ten Dijke P, Lamas S, Rodriguez-Pascual F. Signaling by ALK5 mediates TGF-beta-induced ET-1 expression in endothelial cells: a role for migration and proliferation. J Cell Sci 120: 1256–1266, 2007 [DOI] [PubMed] [Google Scholar]
- 6. Chawla LS, Seneff MG, Nelson DR, Williams M, Levy H, Kimmel PL, Macias WL. Elevated plasma concentrations of IL-6 and elevated APACHE II score predict acute kidney injury in patients with severe sepsis. Clin J Am Soc Nephrol 2: 22–30, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Cheng J, Grande JP. Transforming growth factor-beta signal transduction and progressive renal disease. Exp Biol Med (Maywood) 227: 943–956, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Deelman L, Sharma K. Mechanisms of kidney fibrosis and the role of antifibrotic therapies. Curr Opin Nephrol Hypertens 18: 85–90, 2009 [DOI] [PubMed] [Google Scholar]
- 9. Dennen P, Altmann C, Kaufman J, Klein CL, Andres-Hernando A, Ahuja NH, Edelstein CL, Cadnapaphornchai MA, Keniston A, Faubel S. Urine interleukin-6 is an early biomarker of acute kidney injury in children undergoing cardiac surgery. Crit Care 14: R181, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Donnahoo KK, Meng X, Ayala A, Cain MP, Harken AH, Meldrum DR. Early kidney TNF-α expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am J Physiol Regul Integr Comp Physiol 277: R922–R929, 1999 [DOI] [PubMed] [Google Scholar]
- 11. Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Asano M, Wang H, Matsushima K, Takeya M, Kuziel WA, Mukaida N, Yokoyama H. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J Am Soc Nephrol 14: 2503–2515, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, Ishiwata Y, Tomosugi N, Mukaida N, Matsushima K, Egashira K, Yokoyama H. Gene therapy expressing amino-terminal truncated monocyte chemoattractant protein-1 prevents renal ischemia-reperfusion injury. J Am Soc Nephrol 14: 1066–1071, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Ge Q, Moir LM, Black JL, Oliver BG, Burgess JK. TGFbeta1 induces IL-6 and inhibits IL-8 release in human bronchial epithelial cells: the role of Smad2/3. J Cell Physiol 225: 846–854, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Geng H, Lan R, Wang G, Siddiqi AR, Naski MC, Brooks AI, Barnes JL, Saikumar P, Weinberg JM, Venkatachalam MA. Inhibition of autoregulated TGFbeta signaling simultaneously enhances proliferation and differentiation of kidney epithelium and promotes repair following renal ischemia. Am J Pathol 174: 1291–1308, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Guan Q, Nguan CY, Du C. Expression of transforming growth factor-beta1 limits renal ischemia-reperfusion injury. Transplantation 89: 1320–1327, 2010 [DOI] [PubMed] [Google Scholar]
- 16. Hill-Kapturczak N, Truong L, Thamilselvan V, Visner GA, Nick HS, Agarwal A. Smad7-dependent regulation of heme oxygenase-1 by transforming growth factor-beta in human renal epithelial cells. J Biol Chem 275: 40904–40909, 2000 [DOI] [PubMed] [Google Scholar]
- 17. Hunley TE, Kon V. Endothelin in ischemic acute renal failure. Curr Opin Nephrol Hypertens 6: 394–400, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Ikushima H, Miyazono K. TGFbeta signalling: a complex web in cancer progression. Nat Rev Cancer 10: 415–424, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Jarmi T, Agarwal A. Heme oxygenase and renal disease. Curr Hypertens Rep 11: 56–62, 2009 [DOI] [PubMed] [Google Scholar]
- 20. Juncos JP, Grande JP, Croatt AJ, Hebbel RP, Vercellotti GM, Katusic ZS, Nath KA. Early and prominent alterations in hemodynamics, signaling, and gene expression following renal ischemia in sickle cell disease. Am J Physiol Renal Physiol 298: F892–F899, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Juncos JP, Grande JP, Murali N, Croatt AJ, Juncos LA, Hebbel RP, Katusic ZS, Nath KA. Anomalous renal effects of tin protoporphyrin in a murine model of sickle cell disease. Am J Pathol 169: 21–31, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kielar ML, John R, Bennett M, Richardson JA, Shelton JM, Chen L, Jeyarajah DR, Zhou XJ, Zhou H, Chiquett B, Nagami GT, Lu CY. Maladaptive role of IL-6 in ischemic acute renal failure. J Am Soc Nephrol 16: 3315–3325, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Klein CL, Hoke TS, Fang WF, Altmann CJ, Douglas IS, Faubel S. Interleukin-6 mediates lung injury following ischemic acute kidney injury or bilateral nephrectomy. Kidney Int 74: 901–909, 2008 [DOI] [PubMed] [Google Scholar]
- 24. Kohan DE. Endothelins in the normal and diseased kidney. Am J Kidney Dis 29: 2–26, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Kwon O, Molitoris BA, Pescovitz M, Kelly KJ. Urinary actin, interleukin-6, and interleukin-8 may predict sustained ARF after ischemic injury in renal allografts. Am J Kidney Dis 41: 1074–1087, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Lee HT, Chen SW, Doetschman TC, Deng C, D'Agati VD, Kim M. Sevoflurane protects against renal ischemia and reperfusion injury in mice via the transforming growth factor-β1 pathway. Am J Physiol Renal Physiol 295: F128–F136, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee HT, Kim M, Kim J, Kim N, Emala CW. TGF-beta1 release by volatile anesthetics mediates protection against renal proximal tubule cell necrosis. Am J Nephrol 27: 416–424, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Lee HT, Kim M, Song JH, Chen SW, Gubitosa G, Emala CW. Sevoflurane-mediated TGF-β1 signaling in renal proximal tubule cells. Am J Physiol Renal Physiol 294: F371–F378, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Liu KD, Altmann C, Smits G, Krawczeski CD, Edelstein CL, Devarajan P, Faubel S. Serum interleukin-6 and interleukin-8 are early biomarkers of acute kidney injury and predict prolonged mechanical ventilation in children undergoing cardiac surgery: a case-control study. Crit Care 13: R104, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int 69: 213–217, 2006 [DOI] [PubMed] [Google Scholar]
- 31. Meldrum KK, Meldrum DR, Meng X, Ao L, Harken AH. TNF-α-dependent bilateral renal injury is induced by unilateral renal ischemia-reperfusion. Am J Physiol Heart Circ Physiol 282: H540–H546, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Meng XM, Huang XR, Chung AC, Qin W, Shao X, Igarashi P, Ju W, Bottinger EP, Lan HY. Smad2 protects against TGF-beta/Smad3-mediated renal fibrosis. J Am Soc Nephrol 21: 1477–1487, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meulmeester E, Ten Dijke P. The dynamic roles of TGF-beta in cancer. J Pathol 223: 205–218, 2011 [DOI] [PubMed] [Google Scholar]
- 34. Munshi R, Johnson A, Siew ED, Ikizler TA, Ware LB, Wurfel MM, Himmelfarb J, Zager RA. MCP-1 gene activation marks acute kidney injury. J Am Soc Nephrol 22: 165–175, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nath KA. Heme oxygenase-1: a provenance for cytoprotective pathways in the kidney and other tissues. Kidney Int 70: 432–443, 2006 [DOI] [PubMed] [Google Scholar]
- 36. Nath KA, Croatt AJ, Haggard JJ, Grande JP. Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Nath KA, Grande JP, Croatt AJ, Frank E, Caplice NM, Hebbel RP, Katusic ZS. Transgenic sickle mice are markedly sensitive to renal ischemia-reperfusion injury. Am J Pathol 166: 963–972, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Patel NS, Chatterjee PK, Di Paola R, Mazzon E, Britti D, De Sarro A, Cuzzocrea S, Thiemermann C. Endogenous interleukin-6 enhances the renal injury, dysfunction, and inflammation caused by ischemia/reperfusion. J Pharmacol Exp Ther 312: 1170–1178, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Pittock ST, Norby SM, Grande JP, Croatt AJ, Bren GD, Badley AD, Caplice NM, Griffin MD, Nath KA. MCP-1 is up-regulated in unstressed and stressed HO-1 knockout mice: pathophysiologic correlates. Kidney Int 68: 611–622, 2005 [DOI] [PubMed] [Google Scholar]
- 40. Roberts AB, Tian F, Byfield SD, Stuelten C, Ooshima A, Saika S, Flanders KC. Smad3 is key to TGF-beta-mediated epithelial-to-mesenchymal transition, fibrosis, tumor suppression and metastasis. Cytokine Growth Factor Rev 17: 19–27, 2006 [DOI] [PubMed] [Google Scholar]
- 41. Rodriguez-Pascual F, Redondo-Horcajo M, Lamas S. Functional cooperation between Smad proteins and activator protein-1 regulates transforming growth factor-beta-mediated induction of endothelin-1 expression. Circ Res 92: 1288–1295, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Schnaper HW, Jandeska S, Runyan CE, Hubchak SC, Basu RK, Curley JF, Smith RD, Hayashida T. TGF-beta signal transduction in chronic kidney disease. Front Biosci 14: 2448–2465, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shiraishi F, Curtis LM, Truong L, Poss K, Visner GA, Madsen K, Nick HS, Agarwal A. Heme oxygenase-1 gene ablation or expression modulates cisplatin-induced renal tubular apoptosis. Am J Physiol Renal Physiol 278: F726–F736, 2000 [DOI] [PubMed] [Google Scholar]
- 44. Simmons EM, Himmelfarb J, Sezer MT, Chertow GM, Mehta RL, Paganini EP, Soroko S, Freedman S, Becker K, Spratt D, Shyr Y, Ikizler TA. Plasma cytokine levels predict mortality in patients with acute renal failure. Kidney Int 65: 1357–1365, 2004 [DOI] [PubMed] [Google Scholar]
- 45. Spurgeon KR, Donohoe DL, Basile DP. Transforming growth factor-β in acute renal failure: receptor expression, effects on proliferation, cellularity, and vascularization after recovery from injury. Am J Physiol Renal Physiol 288: F568–F577, 2005 [DOI] [PubMed] [Google Scholar]
- 46. Tanaka T, Nangaku M. The role of hypoxia, increased oxygen consumption, and hypoxia-inducible factor-1 alpha in progression of chronic kidney disease. Curr Opin Nephrol Hypertens 19: 43–50, 2010 [DOI] [PubMed] [Google Scholar]
- 47. Tracz MJ, Juncos JP, Croatt AJ, Ackerman AW, Grande JP, Knutson KL, Kane GC, Terzic A, Griffin MD, Nath KA. Deficiency of heme oxygenase-1 impairs renal hemodynamics and exaggerates systemic inflammatory responses to renal ischemia. Kidney Int 72: 1073–1080, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tracz MJ, Juncos JP, Grande JP, Croatt AJ, Ackerman AW, Rajagopalan G, Knutson KL, Badley AD, Griffin MD, Alam J, Nath KA. Renal hemodynamic, inflammatory, and apoptotic responses to lipopolysaccharide in HO-1−/− mice. Am J Pathol 170: 1820–1830, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK. Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zager RA, Johnson AC, Hanson SY, Lund S. Ischemic proximal tubular injury primes mice to endotoxin-induced TNF-α generation and systemic release. Am J Physiol Renal Physiol 289: F289–F297, 2005 [DOI] [PubMed] [Google Scholar]
- 51. Zhang D, Sun L, Xian W, Liu F, Ling G, Xiao L, Liu Y, Peng Y, Haruna Y, Kanwar YS. Low-dose paclitaxel ameliorates renal fibrosis in rat UUO model by inhibition of TGF-beta/Smad activity. Lab Invest 90: 436–447, 2010 [DOI] [PubMed] [Google Scholar]