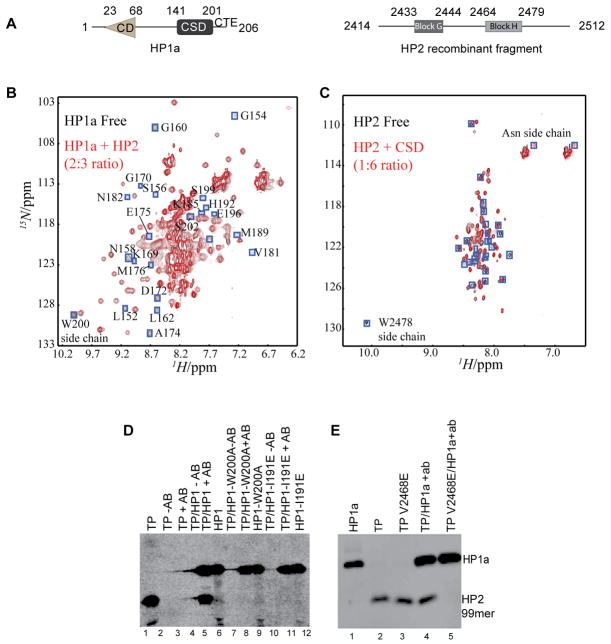
Figure 1. The disordered HP2 fragment binds to the HP1a CSD using a critical valine.
A. HP1a and the HP2 recombinant fragment from D. melanogaster used here are diagrammed. HP1a is made up of a chromodomain (CD) linked to a chromo shadow domain (CSD) by a flexible linker; the C-terminal extension (CTE) is also indicated. In HP2, blocks G and H represent regions conserved among D. melanogaster; D. pseudoobscura; D. willistoni; and D. virilis. B and C. 2D [15N-1H] HSQC spectra demonstrate the specific interaction of the CSD with the HP2 recombinant fragment from A. Resonances that disappear after titration are boxed (blue). In panel B, the 15N-labeled full length HP1a protein is shown in the absence (black) and presence (red) of the HP2 recombinant fragment; the resulting chemical shift perturbations all map to the CSD and CTE, not to the CD or hinge. In C, the 15N labeled HP2 recombinant fragment is shown in the absence (black) and presence (red) of the CSD [residues 131–203 as defined by [23]]. The HP2 fragment appears disordered. D and E. The CSD complex with the HP2 fragment is disrupted by mutations W200A and I191E in HP1a or by mutation V2468E in HP2. Antibody WA192 (specific for HP1a) was used to immunoprecipitate S-35 labeled HP1a and the bound fragment; the immunoprecipitated proteins were resolved by SDS-PAGE and detected by autoradiography. TP corresponds to the test peptide, the HP2 recombinant fragment (seen in A).