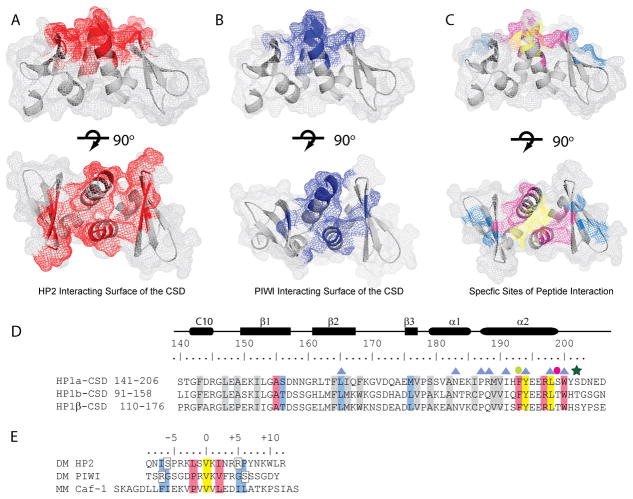
Figure 5. HP2 utilizes an extensive peptide binding surface in the CSD.
A and B. Residues are highlighted that are perturbed more than 0.1 ppm in the complex of the CSD with HP2 (A. Red) and PIWI (B. Blue). The upper panels show a side view; rotating the structures 90° about the horizontal axis shows the top view of the peptide binding surface in the lower panels. C. Residues in the CSD structure are highlighted according to the color scheme in D. for yellow, blue, and pink. D. Multiple sequence alignment of the Drosophila HP1a and HP1b with mouse HP1β. Highlighted in yellow are the residues that interact with valine at position 0 of the interacting peptide. Residues that interact with the +2/−2 position are pink, those that interact in the −6/−7 and +5/+6 positions are blue, and those important for the fold of the CSD are grey. Blue triangles denote residues that are important for dimerization. The green dot denotes a residue that is important for both the fold of the CSD and for peptide binding. The pink dot is a residue important for the fold of the CSD which can also be phosphorylated in HP1a; the green star is a second phosphorylation site. D. Alignment of the HP2, PIWI, and mouse Caf-1 peptides that interact with the CSD. Residues are highlighted in colors that correspond to their interaction sites with the CSD (shown in C). Yellow denotes the central valine, pink the +2/−2 positions and blue the −7/−6 and +5/+6 positions previously determined to be important for binding.