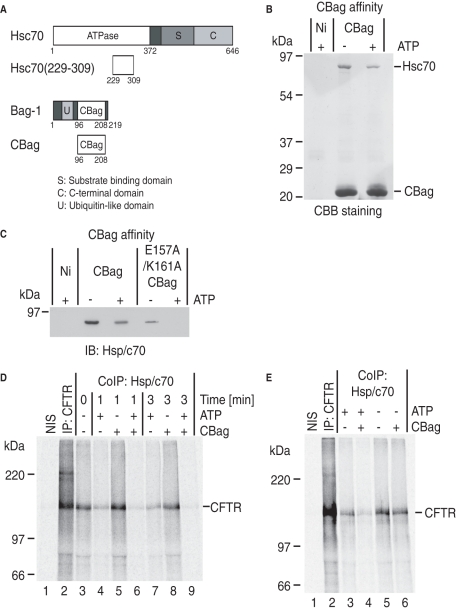
FIGURE 2:
CBag destabilizes Hsc70-CFTR interactions. (A) Schematic representation of Hsc70, Hsc70(229–309), Bag-1, and CBag. The peptide Hsc70(229–309) is the minimal CBag binding subdomain. (B) CBB staining of CBag affinity pull-down of RRL in the presence and absence of ATP. The predominant interacting partner in RRL is Hsc70. (C) Hsc70 immunoblot of wild type and E157A/K161A CBag pull-down from RRL showing ATP-dependent binding to CBag and reduced binding of mutant CBag. (D) Isolated microsomes containing newly synthesized CFTR were incubated with or without CBag and ATP for 1 or 3 min and immediately solubilized in Triton X-100. Samples were immunoprecipitated with nonimmune sera (NIS, lane 1), anti-CFTR antisera (lane 2), or anti-Hsp/c70 antisera (lanes 3–9). CBag + ATP decreased Hsc70 binding (lanes 6 and 9) compared with ATP or CBag alone. (E) Following CFTR synthesis, CBag was added to the translation reaction +/– hexokinase (to deplete ATP) for 10 min, and microsomes were collected, solubilized, and immunoprecipitated with NIS, anti-CFTR antisera (lane 2), or anti-Hsp/c70 antisera. Incubation with CBag reduced Hsc70 binding in an ATP-dependent manner.