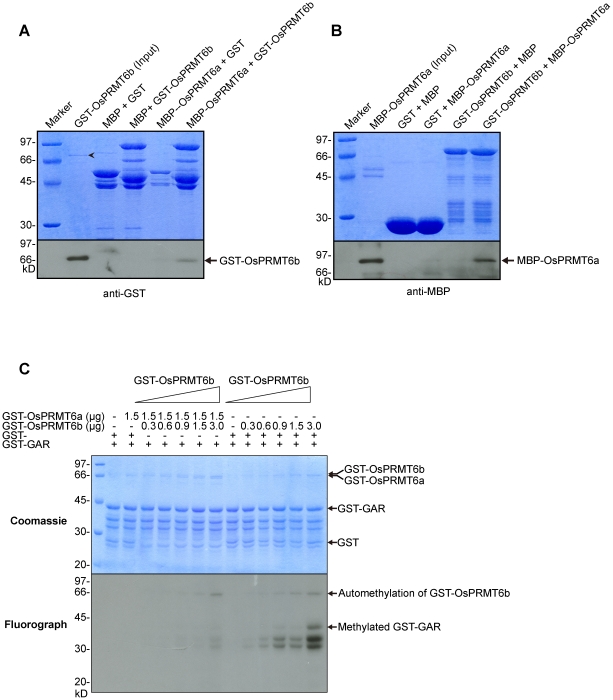
Figure 5. OsPRMT6a and OsPRMT6b directly interact in vitro.
(A and B) GST, MBP, MBP-OsPRMT6a and GST-OsPRMT6b were expressed in E. coli. Reciprocal pull-down assay was performed as described in the experimental part. Equal amount of each mixture of proteins was separated on two separate 10% SDS-PAGE, one for Coomassie staining (upper panels A and B) for equal loading and the other for western blotting (lower panels A and B). GST and MBP alone were used as negative controls. The protein pairs are indicated at the top of the upper panels. MBP-OsPRMT6a served as bait and GST-OsPRMT6b as prey (A) and vice versa (B). A 10 µl cell extract from E. coli containing MBP-OsPRMT6a and GST-OsPRMT6b was used as input. Western blotting using GST and MBP monoclonal antisera revealed the interaction between OsPRMT6a and OsPRMT6b. The arrow head “???” indicates MBP-OsPRMT6a. (C) 0.3, 0.6, 0.9, 1.5 and 3.0 µg of GST-OsPRMT6b was mixed either with 1.5 µg of GST-OsPRMT6a or with GST protein alone (as control) in an in vitro methyltransferase activity assay as described in figure 3. The Coomassie blue stained and dried gel was exposed at −80°C for four days.