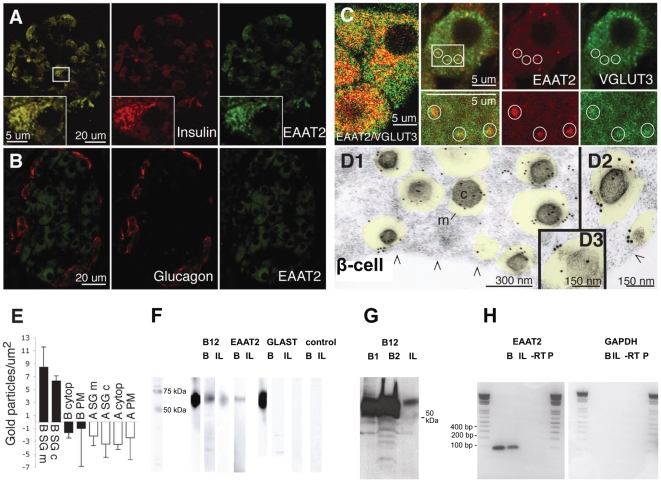
Figure 3. The glutamate transporter EAAT2 is selectively localized in β-cell secretory granules.
(A–B) EAAT2 (green) co-localizes with insulin (red in A) in β-cells but not with glucagon in α-cells (red B). (C) EAAT2 (red) co-localizes partly with the vesicular glutamate transporter VGLUT3 (green). At higher magnification (right panels) it is evident that there are granules containing both EAAT2 and VGLUT3, some of which are indicated by circles. (D1–D3) Electron micrographs showing that EAAT2 immunogold particles are localized in secretory granules (transparent yellow) in three different β-cells. m, granule membrane. c, granule core. Arrowheads (/\), plasma membrane. E, Quantification of EAAT2 in different cellular compartments in α- and β-cells. The values are mean number of EAAT2 gold particles/µm2±SD in the various tissue compartments in 7 α- and 7 β-cells. The EAAT2 density is significantly higher in the membrane (B SG m) and core (B SG c) of the granules in β-cells, than in the cytosol of α- and β-cells (A cyto and B cyto), the plasma membrane of α- and β-cells (A PM and B PM) and the core (A SG c) and limiting membrane (A SG m) of α-cell granules (p<0.001, Mann-Whitney-U test, two tails). (Background labelling was subtracted, see Methods. Except for B SG m and B SG c, all other compartments observed were at background levels.) The quantitative data presented are from one animal and similar results were obtained in two other animals. F, Western blots of isolated rat pancreatic islets (I) and rat brain tissue (B) using EAAT2 antibodies raised against both the C-terminal (B12) and the N-terminal (monoclonal (M)) parts. EAAT1 showed no band in islet tissue. Controls (C) without primary antibody showed no band. Note that the Westerns were run with several different homogenate concentrations, so that the lanes to be directly compared could mostly not be on the same membrane, however all membranes were run in the same set of experiments. (G) Western blot of isolated rat pancreatic islets (I), and rat brain tissue (2 separate brains, B1 and B2) all on the same membrane using EAAT2 antibodies raised against the EAAT2 C-terminal (B12). (H) RT-PCR of rat brain tissue (B) and isolated islets (I) in which there was a PCR signal for EAAT2 at about 100 bp (expected value), but not for human GAPDH.