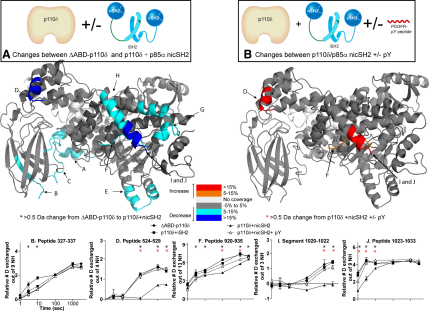
Figure 3.
Changes in Deuteration Levels of p110δ Catalytic Subunit in the Presence of Both p85α and PDGFR pY
(A) Peptides spanning p110δ (labeled A–I) that showed >0.5 Da changes in deuteration level in the presence and absence of the p85α nicSH2 are mapped onto the ΔABD-p110δ structure (2WXH) according to the legend. Peptides that showed >10% change for more than two time points are graphed and shown below the figure. Experiments were performed in duplicate, and graphs are shown ± SD. All other peptides with changes >0.5 Da are shown in Figure S4.
(B) Peptides spanning the p110δ catalytic subunit in the p110δ+nicSH2 complex that showed >0.5 Da changes in deuteration level in the presence and absence of 40 μM PDGFR pY are mapped onto the structure. The percent change mapped on the structure according to the legend is the highest deuterium exchange difference change seen at any time point in the analysis. The area from 1020–1022 is named a segment to denote that this data was generated by subtraction of the deuterium level of peptide 1001–1019 from peptide 1001–1022. The graphs are labeled (∗) for any time points with a >0.5 Da change between the ΔABD-p110δ and p110δ+nicSH2 constructs and (∗) for any time point with a >0.5 Da change for the p110δ+nicSH2 +/− PDGFR pY (see also Figure S4).