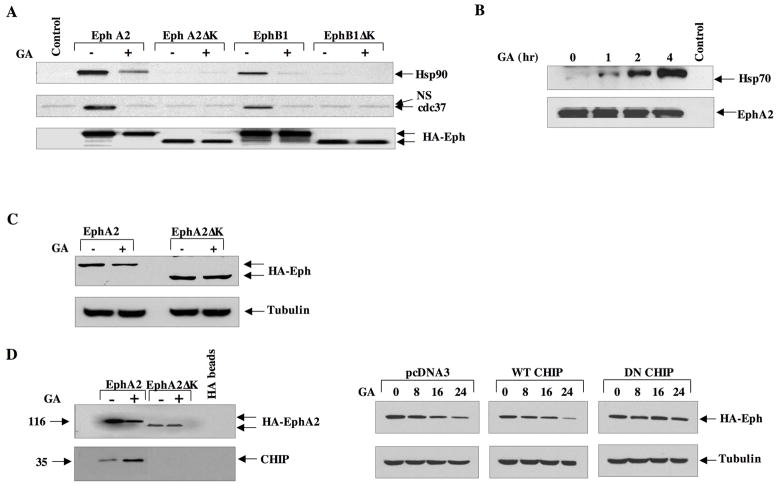
Fig 2. The kinase domain of EphA2 is required for chaperone recruitment and drug sensitivity.
(A) HEK293 cells were cultured in 10 cm dishes and transfected with 5 ug of the indicated plasmids. Following lysis, 1 mg total lysate was immunoprecipitated with HA-conjugated protein G beads and the blots probed for the indicated proteins. (B) PC3 cells were treated with 1 μM GA for the indicated times, EphA2 was immunoprecipitated as in A, and resultant blots were incubated with antibodies to either Hsp70 or EphA2. C) HEK293 cells were transfected with either full length or kinase deleted HA-tagged EphA2, treated for 16 h with GA, and equivalent protein was immunoblotted with HA antibody. D) (Left panel) HEK293 cells were transfected as in C, treated with GA for 8 h, and 1 mg total protein was immunoprecipiated as in A. Resultant blots were probed for CHIP and EphA2 expression (anti-HA). (Right panel) HEK293 cells were transfected with either pcDNA3.1 control vector, wild type CHIP, or dominant negative CHIP, and EphA2 levels were immunodetected as indicated.