Table 1.
Base Screena
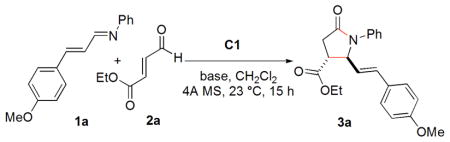 | ||||
|---|---|---|---|---|
| entry | base | yield (%)b | drc | ee (%)d |
| 1 | Et3N | 39 | 9:1 | 14 |
| 2e | Et3N | trace | --- | --- |
| 3 | KHMDS | 25 | 5/1 | 23 |
| 4 | NaOAc | 62 | 5/1 | 42 |
| 5 | PhCOONa | 72 | 6/1 | 50 |
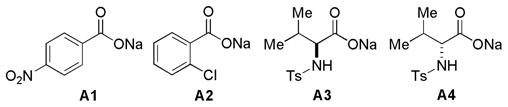
| 6 | A1 | 94 | 5/1 | 57 |
| 7 | A2 | 94 | 5/1 | 59 |
| 8 | A3 | 76 | 6/1 | 33 |
| 9 | A4 | 80 | 6/1 | 41 |
| ||||
Conditions: 2a, 0.2 mmol; 1a, 0.1 mmol; base, 20 mol%; C1 (structure in Table 2), 20 mol%; CH2Cl2, 1 mL; 4A MS under Ar.
NMR yields of trans and cis-diastereomers (internal standard).
The ratio of trans/cis determined by 1H NMR.
Ee of trans-3a determined by chiral HPLC.
1.2 eq of Et3N was used.
