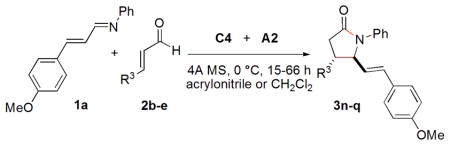
Table 4.
Exploration of Various Enalsa
 | |||||
|---|---|---|---|---|---|
| entry | R3 | product | yield (%) | dr | ee (%) |
| 1b |
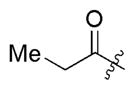 2b 2b
|
3n | 62 | 11/1 | 66 |
| 2c | 4-NO2-C6H4 2c | 3o | 99 | 14/1 | 93 |
| 3d | 4-Br-C6H4 2d | 3p | 58 | 17/1 | 91 |
| 4d | Ph 2e | 3q | 48 | 20/1 | 90 |
Conditions: aldehyde 2, 0.2 mmol; 1a, 0.1 mmol; A2, 20 mol%; C4; 20 mol%; solv., 0.8 mL; all reactions were carried out under Ar in the presence of 4A MS at 0 °C. The trans/cis ratio is determined by 1H NMR prior to purification. The ee’s are determined by chiral HPLC.
15 h in acrylonitrile.
36 h in CH2Cl2.
66 h in CH2Cl2.
