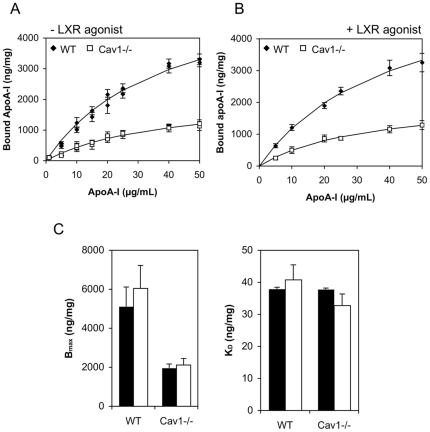
Figure 2. ApoA-I binding to WT and Cav1−/− MEFs at 4°C.
A.–B. 125Iodine-labeled apoA-I (0–50 µg/mL) was bound to WT (solid diamonds) and Cav1−/− MEFs (open squares) at 4°C on ice. Prior to exposure to apoA-I, cells in B, but not A, were treatment with the LXR agonist T0901317. Specific binding was determined by competition with 10-fold excess of unlabeled apoA-I. Specifically bound apoA-I was normalized to cell protein. The data (symbols) are a representative of independent experiments each performed in triplicate cell cultures. Solid lines represent best fits as described in Methods. C. The number of maximum binding sites, Bmax and the binding affinity parameter KD (− LXR agonist shown in filled bars, + LXR agonist hollow bars) obtained from the fit are shown as average ± range of two independent experiments each performed in triplicates. No difference (P>0.05) was found ± LXR agonist treatment.