Abstract
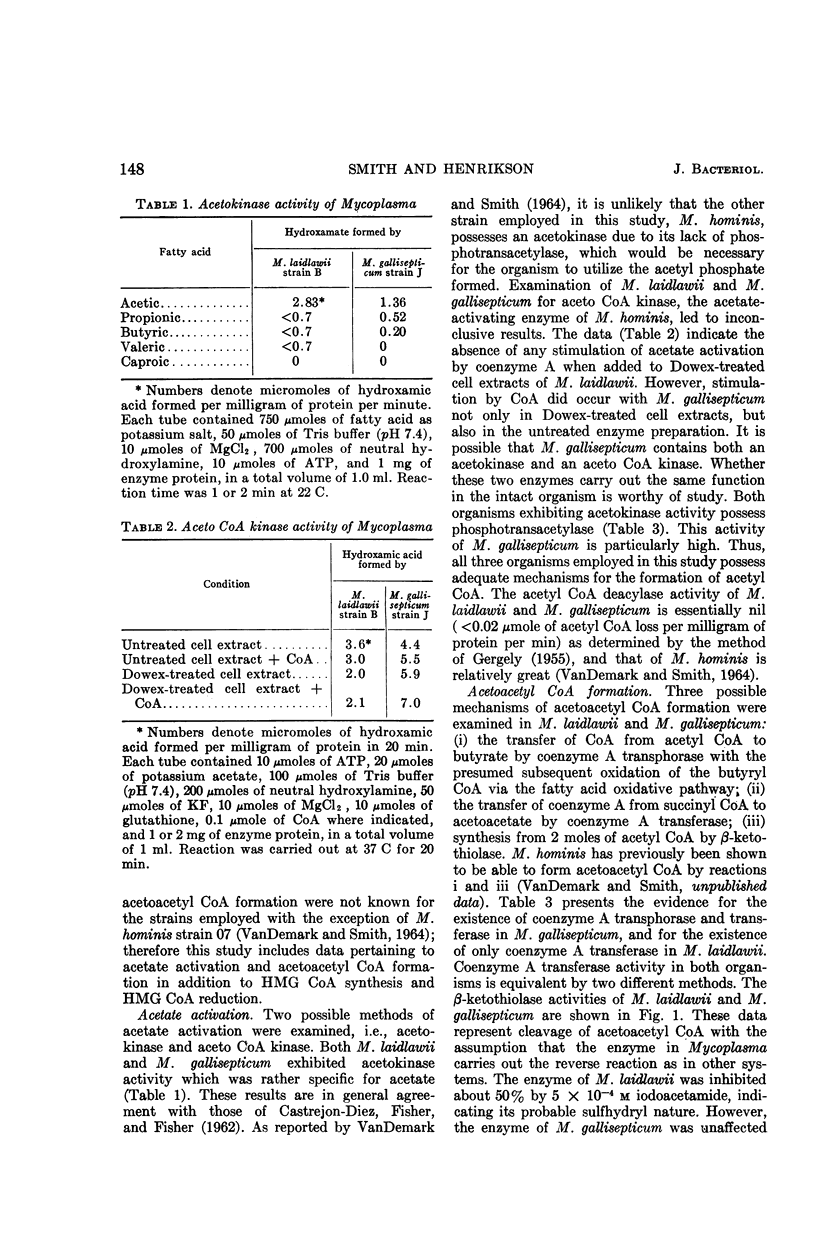
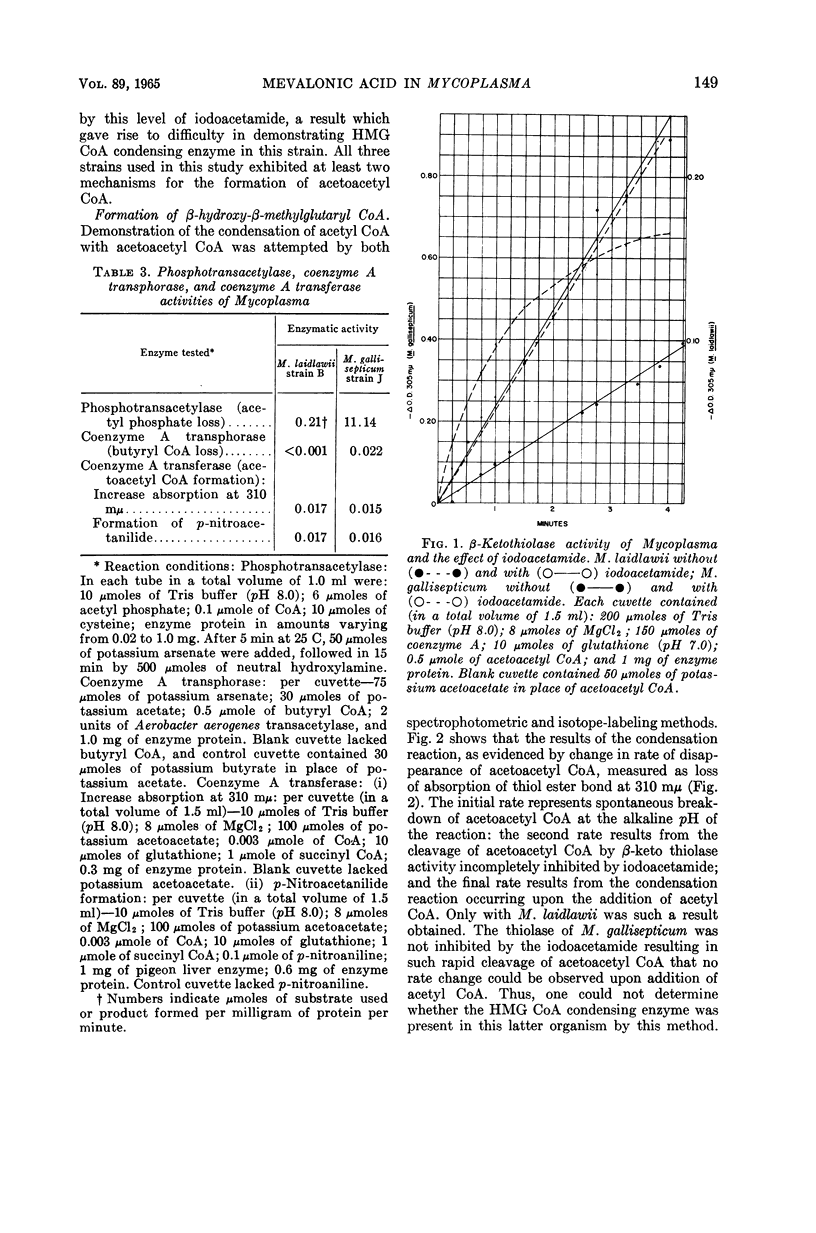
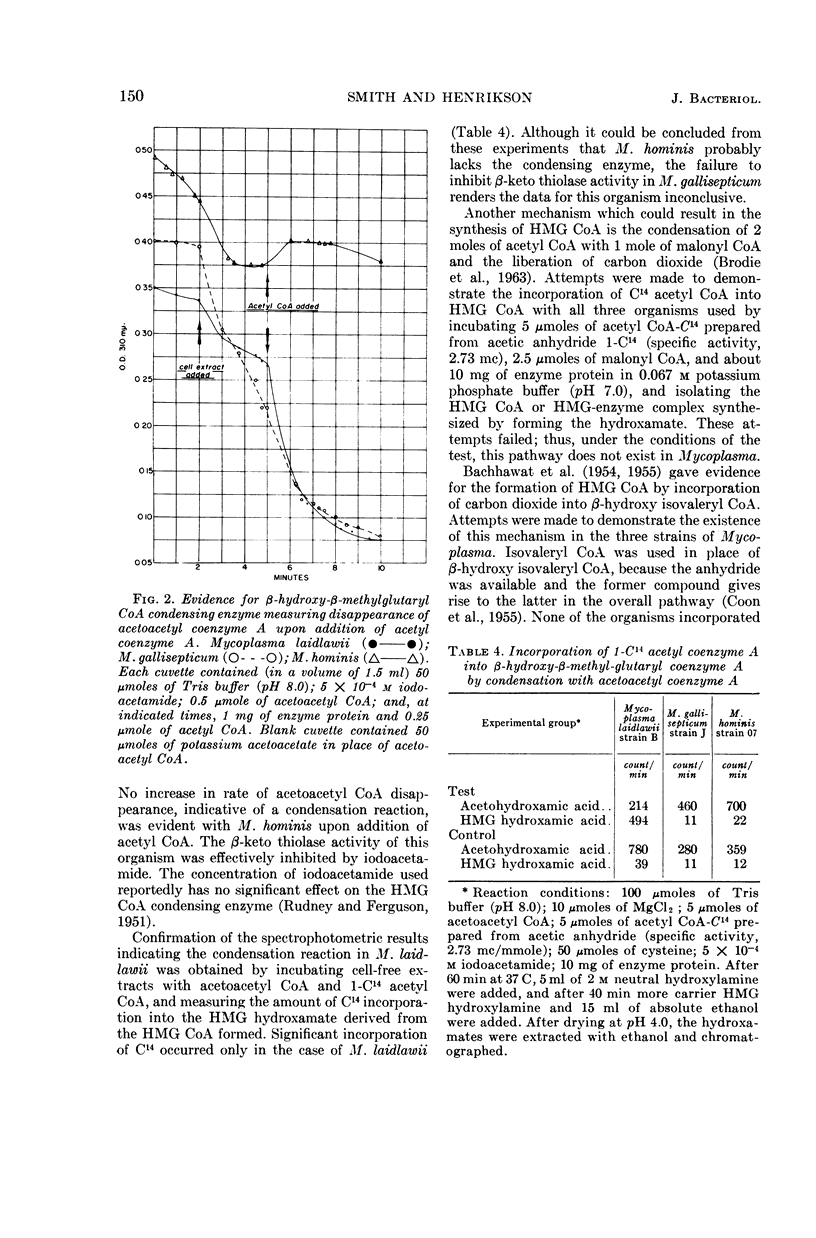
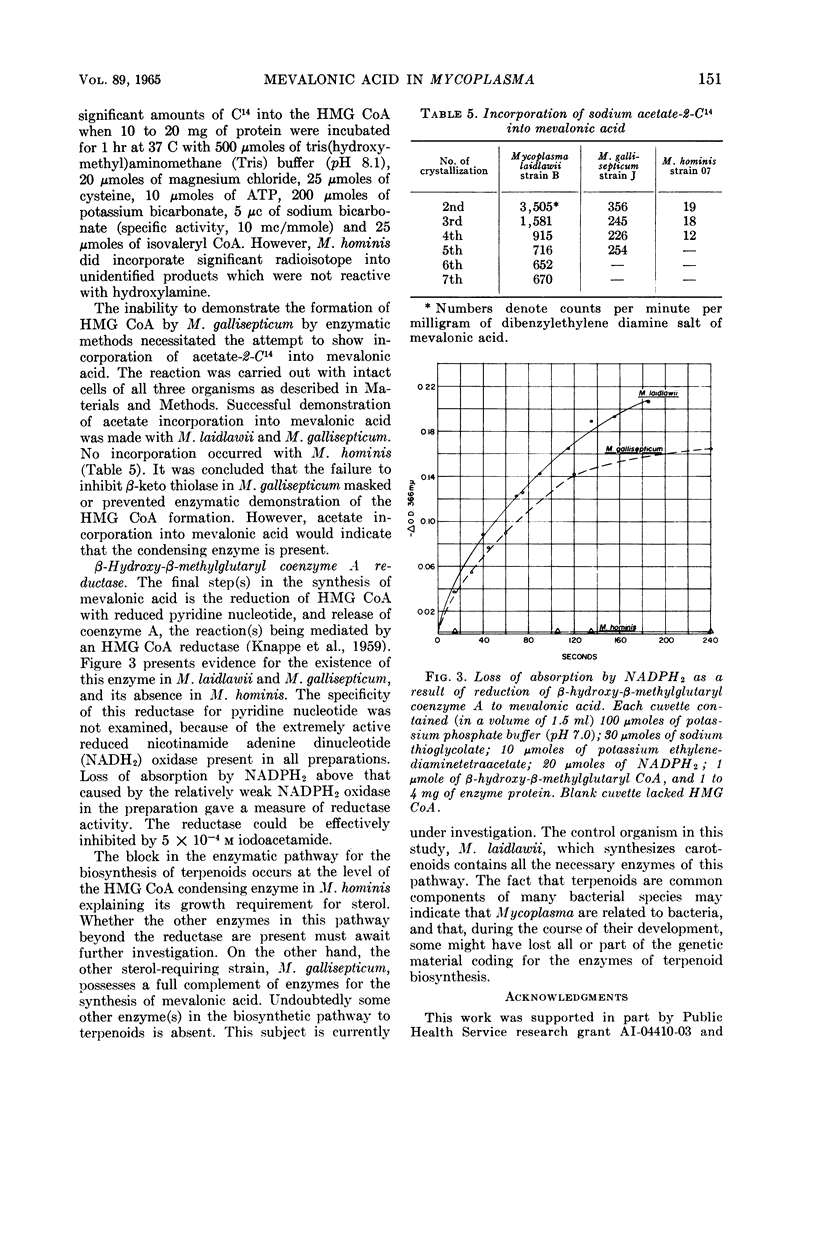
Smith, Paul F. (University of South Dakota, Vermillion), and C. V. Henrikson. Comparative biosynthesis of mevalonic acid by Mycoplasma. J. Bacteriol. 89:146–153. 1965.—Three representative Mycoplasma, M. laidlawii strain B, M. gallisepticum strain J, and M. hominis strain 07, were examined for the presence or absence of enzymes associated with the biosynthetic pathway to mevalonic acid. M. laidlawii served as a control, because it synthesizes carotenoids from acetate. M. laidlawii was shown to contain a specific acetokinase and phosphotransacetylase for the synthesis of acetyl coenzyme A, and a β-ketothiolase and coenzyme A transferase for the synthesis of acetoacetyl coenzyme A. M. gallisepticum contained a specific acetokinase, phosphotransacetylase, and possibly an aceto coenzyme A kinase forming acetyl coenzyme A; it also contained a β-ketothiolase, a coenzyme A transferase, and a coenzyme A transphorase forming acetoacetyl coenzyme A directly or indirectly. The β-ketothiolase of M. gallisepticum was not affected by iodoacetamide, in contrast to the other two strains. M. laidlawii exhibited β-hydroxy-β-methylglutaryl coenzyme A condensing enzyme, and M. hominis did not. This activity of M. gallisepticum was masked by thiolase activity. M. laidlawii and M. gallisepticum contained a nicotinamide adenine dinucleotide phosphate-linked β-hydroxy-β-methylglutaryl coenzyme A reductase, and M. hominis did not. C14-labeled acetate was incorporated into mevalonic acid only by M. laidlawii and M. gallisepticum. The lack of β-hydroxy-β-methylglutaryl coenzyme A condensing enzyme and reductase activities in M. hominis explains its growth requirement for sterol. The enzymatic block in M. gallisepticum must occur after mevalonic acid in the biosynthetic pathway to terpenoids.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., PORTER J. W. The biosynthesis of phytoene and other carotenes by enzymes of isolated higher plant plastids. Arch Biochem Biophys. 1962 Jun;97:509–519. doi: 10.1016/0003-9861(62)90115-7. [DOI] [PubMed] [Google Scholar]
- BACHHAWAT B. K., ROBINSON W. G., COON M. J. The enzymatic cleavage of beta-hydroxy-beta-methylglutaryl coenzyme A to acetoacetate and acetyl coenzyme A. J Biol Chem. 1955 Oct;216(2):727–736. [PubMed] [Google Scholar]
- BRODIE J. D., WASSON G., PORTER J. W. The participation of malonyl coenzyme A in the biosynthesis of mevalonic acid. J Biol Chem. 1963 Apr;238:1294–1301. [PubMed] [Google Scholar]
- CARR N. G. Enzymes of the beta-hydroxy-beta-methylglutaryl-coenzyme A cycle in Rhodopseudomonas spheroides. Biochim Biophys Acta. 1962 Jan 29;56:604–606. doi: 10.1016/0006-3002(62)90615-7. [DOI] [PubMed] [Google Scholar]
- CASTREJON-DIEZ J., FISHER T. N., FISHER E., Jr Acetokinase reaction in several pleuropneumonia-like organisms. Biochem Biophys Res Commun. 1962 Nov 27;9:416–420. doi: 10.1016/0006-291x(62)90026-8. [DOI] [PubMed] [Google Scholar]
- EDWARD D. G., KANAREK A. D. Organisms of the pleuropneumonia group of avian origin: their classification into species. Ann N Y Acad Sci. 1960 Jan 15;79:696–702. doi: 10.1111/j.1749-6632.1960.tb42744.x. [DOI] [PubMed] [Google Scholar]
- HILZ H., KNAPPE J., RINGELMANN E., LYNEN F. Methylglutaconase, eine neue Hydratase, die am Stoffwechsel verzweigter Carbonsäuren beteiligt ist. Biochem Z. 1958;329(6):476–489. [PubMed] [Google Scholar]
- KNAPPE J., RINGELMANN E., LYNEN F. [On beta-hydroxy-beta-methylglutaryl reductase of yeast. On the biosynthesis of terpene. IX]. Biochem Z. 1959;332:195–213. [PubMed] [Google Scholar]
- PORTER J. W., ANDERSON D. G. The biosynthesis of carotenes. Arch Biochem Biophys. 1962 Jun;97:520–528. doi: 10.1016/0003-9861(62)90116-9. [DOI] [PubMed] [Google Scholar]
- ROTHBLAT G. H., SMITH P. F. Nonsaponifiable lipids of representative pleuropneumonia-like organisms. J Bacteriol. 1961 Oct;82:479–491. doi: 10.1128/jb.82.4.479-491.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. COMPARATIVE PHYSIOLOGY OF PLEUROPNEUMONIA-LIKE AND L-TYPE ORGANISMS. Bacteriol Rev. 1964 Jun;28:97–125. doi: 10.1128/br.28.2.97-125.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F., LYNN R. J. Lipid requirements for the growth of pleuropneumonia-like organisms. J Bacteriol. 1958 Sep;76(3):264–269. doi: 10.1128/jb.76.3.264-269.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F., ROTHBLAT G. H. Comparison of lipid composition of pleuropneumonia-like and L-type organisms. J Bacteriol. 1962 Mar;83:500–506. doi: 10.1128/jb.83.3.500-506.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMITH P. F. THE CAROTENOID PIGMENTS OF MYCOPLASMA. J Gen Microbiol. 1963 Sep;32:307–319. doi: 10.1099/00221287-32-3-307. [DOI] [PubMed] [Google Scholar]
- STADTMAN E. R., NOVELLI G. D., LIPMANN F. Coenzyme A function in and acetyl transfer by the phosphotransacetylase system. J Biol Chem. 1951 Jul;191(1):365–376. [PubMed] [Google Scholar]
- TABOR H., MEHLER A. H., STADTMAN E. R. The enzymatic acetylation of amines. J Biol Chem. 1953 Sep;204(1):127–138. [PubMed] [Google Scholar]
- TOURTELLOTTE M. E., JENSEN R. G., GANDER G. W., MOROWITZ H. J. LIPID COMPOSITION AND SYNTHESIS IN THE PLEUROPNEUMONIA-LIKE ORGANISM MYCOPLASMA GALLISEPTICUM. J Bacteriol. 1963 Sep;86:370–379. doi: 10.1128/jb.86.3.370-379.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VANDEMARK P. J., SMITH P. F. EVIDENCE FOR A TRICARBOXYLIC ACID CYCLE IN MYCOPLASMA HOMINIS. J Bacteriol. 1964 Dec;88:1602–1607. doi: 10.1128/jb.88.6.1602-1607.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


