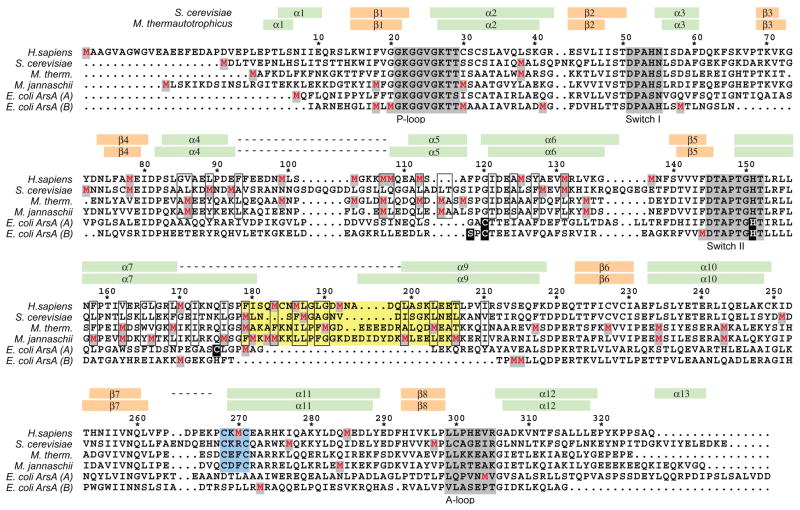
Figure 2. Sequence alignment of eukaryotic and archaeal TRC40/Get3 orthologs with the functionally distinct bacterial ArsA.
Numbering is according to M. thermautotrophicus TRC40. Secondary structure elements (green, orange) and disordered regions (dashed lines) are indicated for MtTRC40 and ScGet3. The four conserved ATPase sequence motifs are highlighted in grey. The ‘TRC40-insert’ (yellow) and the zinc-binding ‘CXXC motif’ (blue) are indicated. Hydrophobic residues lining the MtTRC40 composite groove are boxed. Methionine residues are in red; E. coli ArsA residues involved in antimony binding are in white.