Figure 2.
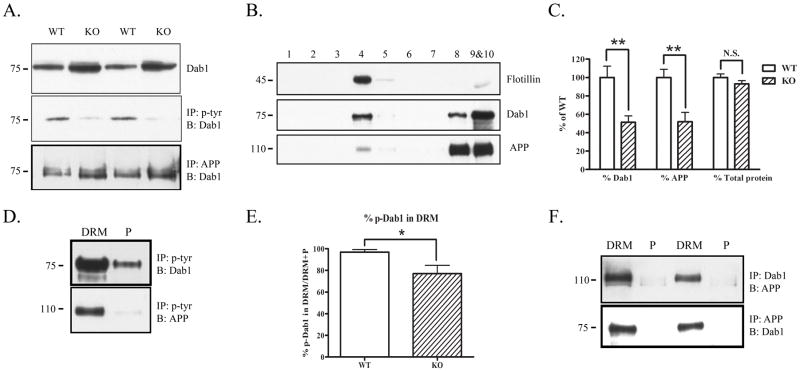
Fyn knock-out mice have decreased APP and Dab1 in DRMs. A. Brain homogenates from wild-type or Fyn knock-out mice were blotted for Dab1 (top panel), immunoprecipitated with phosphotyrosine and blotted for Dab1 (middle panel), or immunoprecipitated with APP (antibody C1/6.1) and blotted for Dab1 (bottom panel). Fyn knock-out mice had increased total Dab1 (top panel) and decreased phosphorylated Dab1 (middle panel) compared to wild-type mice. Fyn knock-out mice had increased co-precipitation of APP and Dab1 compared to wild-type mice (bottom panel). B. Brain homogenates from Fyn knock-out mice were fractionated and probed for Flotillin to demonstrate presence of DRMs in fraction 4 (top panel). Dab1 (middle panel) and APP (bottom panel) were present both in (fraction 4) and out (fractions 8–10) of DRMs in Fyn knock-out mice. C. The ratio of Dab1 levels in DRM fractions of Fyn knock-out mice (normalized to total Dab1 in all fractions) was decreased by 49% (left, p<0.01), and the ratio of APP levels in DRM fractions (normalized to total APP in all fractions) was decreased by 48% (middle, p<0.01) compared to wild-type mice. There was no significant difference between wild-type and knock-out mice in the ratio of total protein found in the DRM fractions (right). D. DRM or pellet fractions from Fyn knock-out mice were immunoprecipitated with phosphotyrosine and blotted for Dab1 or APP. Phosphorylated Dab1 was present out of the DRM fraction in Fyn knock-out mice (top panel). Phosphorylated APP was present only in the DRM fraction as in wild-type mice (bottom panel). E. The ratio of tyrosine phosphorylated Dab1 in DRMs (normalized to the sum of p-Dab1 from DRM and non-DRM fractions) was significantly decreased in Fyn knock-out mice by 21% (p<0.05). F. DRM or pellet fractions from Fyn knock-out mice were immunoprecipitated with Dab1 and blotted with antibody 369 for APP (top panel), or immunoprecipitated with APP and blotted for Dab1 (bottom panel). Dab1 and APP co-precipitated only in DRM fractions.