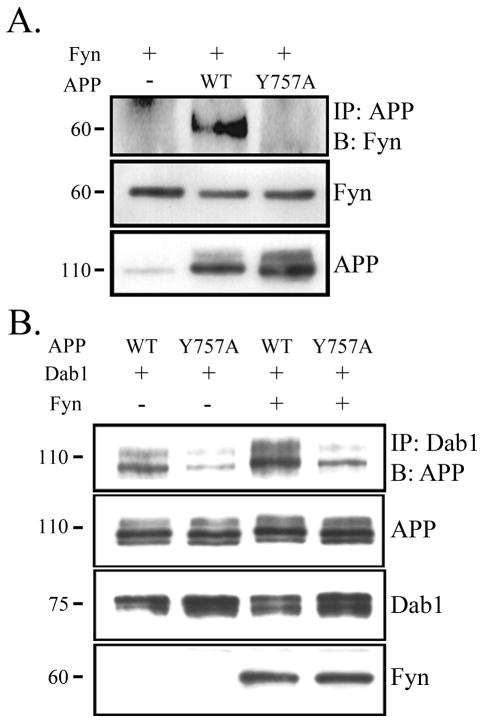
Figure 4.
Phosphorylation of APP increases interaction with Dab1. A. COS7 cells were transfected with Fyn and vector, APP wild-type (WT), or APP mutant construct (Y757A) that contained a substitution mutation at the site of Fyn phosphorylation. Cell lysates were immunoprecipitated with APP and blotted for Fyn (top panel). APP Y757A co-precipitated less with Fyn compared to APP WT. Levels of Fyn and APP remained the same across conditions (bottom panels). B. COS7 cells were transfected with Dab1 and APP WT or APP Y757A in the presence or absence of Fyn. Cell lysates were immunoprecipitated with Dab1 and blotted for APP (top panel). APP Y757A co-precipitated less with Dab1 compared to APP WT both in the presence (right lanes) and absence (left lanes) of Fyn. Levels of APP, Dab1, and Fyn remained similar across conditions (bottom panels).