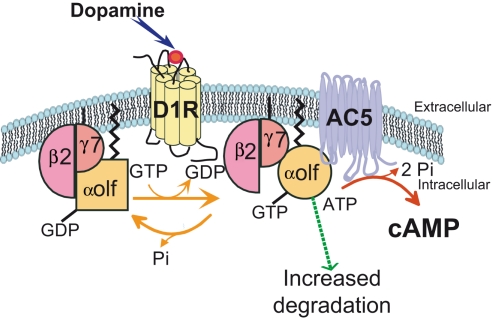
Figure 2.
A specific assembly of Gαolf, Gβ2, and Gγ7 subunits of G protein mediates the coupling of D1 receptor to adenylyl cyclase 5. The expression of Gγ7 subunit in striatal neurons recruits and stabilizes Gαolf and Gβ2 subunits. They form a specific heterotrimeric protein that provides the signaling complex necessary for the coupling of D1R receptor to the adenylyl cyclase 5 (AC5), an isoform particularly enriched in the striatum. The D1R stimulation by dopamine activates the Gαolf/β2/γ7 heterotrimer by triggering substitution of GDP by GTP in the Gαolf subunit and changes in the subunit conformation. The current data indicate that the Gα activation does not necessary cause its dissociation from Gβγ complex as it was thought previously (Bunemann et al., 2003). It has been proposed a “clamshell” model according to which activated receptor provokes movements in Gαβγ complex that unmask previously buried interfaces and enable interaction of Gα and Gβγ with specific effectors (Robishaw and Berlot, 2004). In this model, the Gβγ subunits are not shared among several α subunits but can remain associated with a specific pool of α subunit (Robishaw and Berlot, 2004). Such stable association may contribute to the specificity of Gαolf interaction with Gβ2γ7 complex. In addition, the activation of the heterotrimeric complex could increase its vulnerability to degradation processes. This effect could explain why the receptor usage reduces the Gαolf levels in striatal neurons.