Abstract
Stroke is the leading cause of disability and the third leading cause of death in adults worldwide1. In human stroke, there exists a highly variable clinical state; in the development of animal models of focal ischemia, however, achieving reproducibility of experimentally induced infarct volume is essential. The rat is a widely used animal model for stroke due to its relatively low animal husbandry costs and to the similarity of its cranial circulation to that of humans2,3. In humans, the middle cerebral artery (MCA) is most commonly affected in stroke syndromes and multiple methods of MCA occlusion (MCAO) have been described to mimic this clinical syndrome in animal models. Because recanalization commonly occurs following an acute stroke in the human, reperfusion after a period of occlusion has been included in many of these models. In this video, we demonstrate the transient endovascular suture MCAO model in the spontaneously hypertensive rat (SHR). A filament with a silicon tip coating is placed intraluminally at the MCA origin for 60 minutes, followed by reperfusion. Note that the optimal occlusion period may vary in other rat strains, such as Wistar or Sprague-Dawley. Several behavioral indicators of stroke in the rat are shown. Focal ischemia is confirmed using T2-weighted magnetic resonance images and by staining brain sections with 2,3,5-triphenyltetrazolium chloride (TTC) 24 hours after MCAO.
Keywords: Neuroscience, Issue 48, Stroke, cerebral ischemia, middle cerebral artery occlusion, intraluminal filament, rat, magnetic resonance imaging, surgery, neuroscience, brain
Protocol
MCAO Rat Model
Presurgical Preparations
Aseptic technique should be used for all survival surgical procedures. Disinfect the surgical work surface with commercial disinfectant and prepare sterile surgical packs of instruments, drapes, gauze, swabs, sutures, and scalpel blades by autoclaving. A surgical mask, hair bonnet and sterile gloves should be worn. A Germinator dry bead sterilizer is also used to resterilize surgical instruments between procedures if multiple rat surgeries will be done during one session. Prewarm a water-jacketed homeothermic blanket and place under an absorbent pad in order to prevent hypothermia of the rat during surgery.
Place spontaneously hypertensive rat (or other rat strain of choice) into an induction chamber and induce anesthesia with 5% isoflurane (anesthesia machine should be set to 1.0 L/min O2 and 1.0 L/min N2O). Lower to 1-2% isoflurane to maintain anesthesia.
Apply Artificial Tears ointment to both eyes.
Shave the throat and left neck region beyond the prospective incision site using clippers (Oster A5 with #10 blade).
Apply Betadine to a gauze pad and disinfect the skin starting from the center of the surgical region, spiraling outward. Rinse with sterile gauze pad containing 70% ethanol, moving pad in a similar pattern. Repeat both steps for a total of three cycles.
Inject 0.2 mL of 0.5% bupivacaine subcutaneously along the prospective incision site.
Place rat in sterile stockinette or cover with a sterile surgical drape.
Transient occlusion of MCA
Under an operating microscope, a ventral midline incision is performed and the superficial fascia is dissected.
Beneath the superficial fascia, there is glandular tissue to the left and three muscles which form a triangle: the sternohyoid, which lies midline over the trachea; the digastric (easily identified with its shiny white tendinous portion); and lastly, the sternomastoid muscle.
Careful sharp and blunt dissection is performed within the triangle to identify the carotid artery (exclusive blunt dissection may be preferred by some to minimize accidental tissue damage). The external, internal, and common carotid arteries (ECA, ICA, CCA) are exposed. The carotid artery is large and visibly pulses. The vagus nerve is seen coursing along the lateral aspect of both common and internal carotid arteries and is sharply dissected off the common and internal carotid arteries.
Two ECA branches are then dissected sharply, the first branch heading medially and the second branch heading laterally. Both branches are cauterized and cut, which allows for greater ease in mobilizing the larger vessels.
The ECA is now dissected more rostrally. The hyoid bone may be encountered and this will limit the extent of rostral dissection. The external carotid artery is tied off as distally as possible with a 6-0 silk suture.
Another 6-0 silk suture is placed loosely around the ECA near the bifurcation with the ICA. Make sure not to occlude the vessel as the intraluminal suture to be used for the occlusion will be going through here at a later step.
Microsurgical clips are placed on common and internal carotid arteries near the bifurcation.
An initial partial arteriotomy is created between the two silk suture ties on the external carotid artery.
Introduce a 2.2 -3.0 cm length of 4-0 monofilament nylon suture, with a kink 2.0 cm from its rounded, silicon-coated tip, into the ECA lumen down toward the CCA where the microsurgical clip is located. These sutures are commercially available (Doccol Corp., Redlands, CA, USA). Cut the remaining portion of the ECA (at the site of the partial arteriotomy) to free the stump and position the stump below the bifurcation of the ECA and ICA; this will more easily allow the intraluminal suture to slide into the ICA.
Tighten the silk suture around the ECA stump to secure the intraluminal nylon suture and prevent bleeding, and then remove the microvascular clip from the ICA. Open the clip slowly before removing it to check for bleeding.
Continue advancing the nylon suture from the ECA to ICA lumen to the middle cerebral artery (MCA). This length is typically 18-20 mm and is the reason for placing a kink in the suture prior to insertion. After a variable length of nylon suture is inserted, resistance may be felt. If this occurs with much of the nylon suture still present outside of the vessel, it indicates that the suture is likely entering the pterygopalatine artery (see note below). Pull back and curve the suture slightly to continue along the ICA, which will run more medially. Additionally, dissection of the origin of the PPA may be done to better visualize the path of the intraluminal filament. Continue to insert the nylon suture until resistance is felt at and after the 2 cm kinked position. At this point, the intraluminal suture has blocked the origin of the MCA. MCA occlusion can be confirmed by monitoring the reduction in regional cerebral blood flow using a laser Doppler flowmeter (see Materials table for one equipment source). Also note that the origin of the pterygopalatine artery off of the ICA can be directly tied off, if preferred, in order to avoid accidental intubation of this vessel with the intraluminal suture.
Start the timer and record occlusion start time.
Remove the microclip from the CCA.
Close the incision (dermis, panniculus carnosus, subcutaneous tissue layers) quickly with 3-0 silk suture (a simple continuous pattern will facilitate reopening for reperfusion) and carefully place the rat in a recovery cage. Check that the cage floor around the nose and mouth are free of bedding material and monitor recovery from anesthesia.
Restoration of MCA blood flow (reperfusion)
Shortly before the occlusion period should end, re-anesthetize the rat, disinfect the incision site with Betadine and 70% ethanol (3 cycles, as before) and reopen the incision by removing the closing sutures.
Place a microclip on the CCA, as before.
Withdraw the occluding suture partway from the ICA until the suture end is visible through the ICA. Do NOT fully remove the suture from the ICA/ECA!
Place a microclip on the ICA above the end of the intraluminal suture.
Completely remove the occluding suture and tightly tie off the ECA stump. Record end of occlusion (reperfusion start) time.
Remove the microclip from the ICA.
Remove the microclip from the CCA.
Moisten the region with several drops of sterile saline and close the incision layers (dermis, panniculus carnosus, subcutaneous tissue) with 3-0 silk suture using a simple interrupted pattern.
Administer 0.05 mg/kg buprenorphine (or other appropriate post-operative analgesic following your institutional guidelines) into the peritoneal space.
Inject 5cc of prewarmed saline intraperitoneally. This will provide hydration during the recovery stage.
Monitor recovery of rat from anesthesia.
After the rat has recovered, test for behavioral indication of infarction by holding the rat by the tail and observing whether the rat can turn to both sides. Curling to one side only is expected. Simple scoring scales can be used to record initial post-infarct behavorial functioning (see Discussion).
Additional injections of buprenorphine (same dosage as above) should be given every 6 to 8 hours for 24 hr of pain relief. Extend analgesia if animal is showing signs of discomfort.
Representative Results
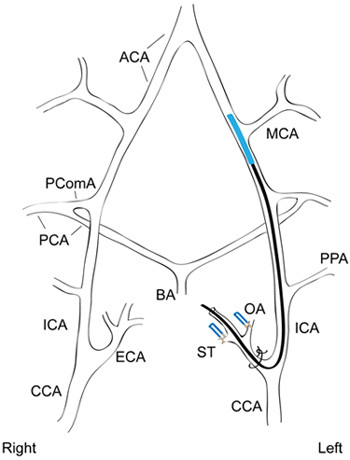 Figure 1. Occlusion of the middle cerebral artery by endovascular suture. A simplified diagram of the cranial circulatory system of the rat is shown with a silicon-coated intraluminal suture occluding the origin of the MCA. The OA and ST branches off of the left ECA have been ligated and a suture tie around the ECA stump holds the intraluminal suture in place. ACA, anterior cerebral artery; BA, basilar artery; CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery; OA, occipital artery; PCA, posterior cerebral artery; PComA, posterior communicating artery; PPA, pterygopalatine artery; ST, superior thyroid artery. Figure adapted from Sasaki et al.4 and Lee3.
Figure 1. Occlusion of the middle cerebral artery by endovascular suture. A simplified diagram of the cranial circulatory system of the rat is shown with a silicon-coated intraluminal suture occluding the origin of the MCA. The OA and ST branches off of the left ECA have been ligated and a suture tie around the ECA stump holds the intraluminal suture in place. ACA, anterior cerebral artery; BA, basilar artery; CCA, common carotid artery; ECA, external carotid artery; ICA, internal carotid artery; MCA, middle cerebral artery; OA, occipital artery; PCA, posterior cerebral artery; PComA, posterior communicating artery; PPA, pterygopalatine artery; ST, superior thyroid artery. Figure adapted from Sasaki et al.4 and Lee3.
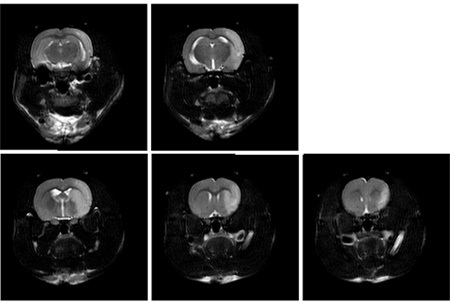 Figure 2. Representative magnetic resonance imaging of coronal sections of rat brain 24 hours after 1 hour transient MCAO. Brain edema accompanying focal ischemia is visualized in T2-weighted MRI images. The infarct zone appears hyperintense (bright) on T2 images. Similar infarct patterns can be seen with more advanced MRI techniques such as diffusion-weighted imaging and apparent diffusion coefficient mapping5.
Figure 2. Representative magnetic resonance imaging of coronal sections of rat brain 24 hours after 1 hour transient MCAO. Brain edema accompanying focal ischemia is visualized in T2-weighted MRI images. The infarct zone appears hyperintense (bright) on T2 images. Similar infarct patterns can be seen with more advanced MRI techniques such as diffusion-weighted imaging and apparent diffusion coefficient mapping5.
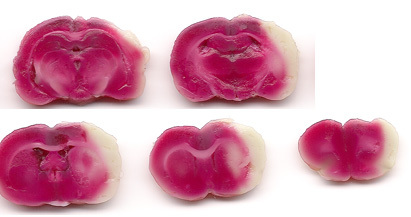 Figure 3. Representative coronal sections of rat brain stained with TTC 24 hours after 1 hour transient MCAO. TTC staining reveals white (unstained) infarcted regions of cerebral cortex and striatum in the same rat as that shown in Figure 2. Note that the general pattern of focal ischemic lesions is similar to that seen in the MRI T2-weighted images, although the final infarct area determined histologically may be slightly smaller than the T2 lesion.
Figure 3. Representative coronal sections of rat brain stained with TTC 24 hours after 1 hour transient MCAO. TTC staining reveals white (unstained) infarcted regions of cerebral cortex and striatum in the same rat as that shown in Figure 2. Note that the general pattern of focal ischemic lesions is similar to that seen in the MRI T2-weighted images, although the final infarct area determined histologically may be slightly smaller than the T2 lesion.
Discussion
Rodent models of cerebral ischemia can be classified as global or focal and as reversible or irreversible. We use a reversible focal ischemia model in order to mimic the reperfusion injury that can occur following recanalization in human stroke patients. While the rat shares the advantage of similar cranial circulation to the human, the extensive intracranial collateral circulation due to the Circle of Willis and leptomeningeal anastomoses results in inconsistent infarct volumes6. We use the spontaneously hypertensive rat (SHR) strain because infarct size is greater in SHRs exposed to focal cerebral ischemia compared to normotensive rats7-10. Stroke induction in SHRs is also more consistent and may generate less mortality than in other commonly used strains11. In addition, physiological parameters should be monitored throughout the surgery, because fluctuations can add to the variability in infarct volumes; these parameters include blood pressure, arterial O2 and CO2 levels, hypothermia, and hyperglycemia, which can exacerbate ischemic injury2,12. Hypothermia is of particular concern because it can lessen the degree of neuronal ischemic injury, and therefore, placing the rat on a thermoregulatory heating pad during surgery is essential.
We employ commercially prepared silicon-coated sutures in this procedure in an attempt to increase consistency in the infarct size13-15. Traditionally, intraluminal sutures used to occlude the MCA have been prepared by flame-rounding a 3-0 nylon suture tip or using a hot glue gun to coat the suture tip with a small glue bead16. These procedures produce sutures with varying tip diameters and thus, may require several attempts at occlusion before finding a suture that matches the vessel diameter of a given rat. Additional variations on the MCAO method described in this video include more distal occlusion, sparing the lenticulostriate branches in order to have a pure cortical infarct17, and simultaneous occlusion of the MCA and ipsilateral common carotid artery to reduce collateral blood flow6,9.
The functional effects of stroke can be assessed using a variety of behavioral tests, in which deficits in specific tasks are reflected in a simple scoring scale. For example, Bederson et al.18 developed a neurological score grading rats on the extent of forelimb flexion in an elevated body position, asymmetric resistance to a lateral push, and open field circling behavior. Rats extending both forelimbs to the floor when elevated and exhibiting no other deficits were graded "Normal (0)", whereas rats with contralateral forelimb flexion only were classified with "Moderate deficits (1)". "Severe grade 2" rats displayed decreased resistance on the contralateral side to a lateral force and "Severe grade 3" rats additionally engaged in circling behaviors. Lower grade behavioral deficits were always observed in the next higher grade (e.g., grade 2 and 3 animals also displayed forelimb flexion) and these scores were predictive of differences in infarct size18. Thus, such a simple scoring system allows for a rapid, consistent semi-quantitative assessment of the functional deficits from stroke.
Measurement of the extent of cerebral infarction is commonly accomplished by histological staining for tissue damage using TTC, cresyl violet (Nissl stain) or hematoxylin and eosin. Magnetic resonance imaging techniques, including diffusion-weighted MRI to visualize the infarct at early time points after occlusion, allow for the determination of an infarct volume in each individual rat without the need for harvesting tissue. This is especially valuable in studies aimed at determining the efficacy of a neuroprotective treatment following stroke; imaging the rat after MCAO but prior to treatment administration gives the investigator confidence that all experimental and control animals experienced similar strokes to start, reducing the confounding effects of variability in stroke induction.
Disclosures
The authors have no conflicting interests to disclose.This protocol was approved by the Institutional Animal Care and Use Committee at University of Wisconsin-Madison School of Medicine and Public Health and abides by the National Institutes of Health guidelines for the use of experimental animals.
Acknowledgments
We thank Beth Rauch and the University of Wisconsin Small Animal Imaging Facility for magnetic resonance imaging. The Department of Neurological Surgery at the University of Wisconsin-Madison provided funding for this work.
References
- van Gijn J, Dennis MS. Issues and answers in stroke care. Lancet. 1998;352(Suppl 3):SIII23–27. doi: 10.1016/s0140-6736(98)90091-5. [DOI] [PubMed] [Google Scholar]
- Macrae IM. New models of focal cerebral ischaemia. British Journal of Clinical Pharmacology. 1992;34:302–302. doi: 10.1111/j.1365-2125.1992.tb05634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee RM. Morphology of cerebral arteries. Pharmacol Ther. 1995;66:149–173. doi: 10.1016/0163-7258(94)00071-a. [DOI] [PubMed] [Google Scholar]
- Sasaki M, Honmou O, Kocsis JD. A rat middle cerebral artery occlusion model and intravenous cellular delivery. Methods Mol Biol. 2009;549:187–195. doi: 10.1007/978-1-60327-931-4_13. [DOI] [PubMed] [Google Scholar]
- Bråtane BT, Bastan B, Fisher M, Bouley J, Henninger N. Ischemic lesion volume determination on diffusion weighted images vs. apparent diffusion coefficient maps. Brain Res. 2009;1279:182–188. doi: 10.1016/j.brainres.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Chen ST, Hsu CY, Hogan EL, Maricq H, Balentine JD. A model of focal ischemic stroke in the rat: reproducible extensive cortical infarction. Stroke. 1986;17:738–743. doi: 10.1161/01.str.17.4.738. [DOI] [PubMed] [Google Scholar]
- Dogan A, Başkaya MK, Rao VL, Rao AM, Dempsey RJ. Intraluminal suture occlusion of the middle cerebral artery in Spontaneously Hypertensive rats. Neurol Res. 1998;20:265–270. doi: 10.1080/01616412.1998.11740517. [DOI] [PubMed] [Google Scholar]
- Ogata J, Fujishima M, Morotomi Y, Omae T. Cerebral infarction following bilateral carotid artery ligation in normotensive and spontaneously hypertensive rats: a pathological study. Stroke. 1976;7:54–60. doi: 10.1161/01.str.7.1.54. [DOI] [PubMed] [Google Scholar]
- Brint S, Jacewicz M, Kiessling M, Tanabe J, Pulsinelli W. Focal brain ischemia in the rat: methods for reproducible neocortical infarction using tandem occlusion of the distal middle cerebral and ipsilateral common carotid arteries. J Cereb Blood Flow Metab. 1988;8:474–485. doi: 10.1038/jcbfm.1988.88. [DOI] [PubMed] [Google Scholar]
- Duverger D, MacKenzie ET. The quantification of cerebral infarction following focal ischemia in the rat: influence of strain, arterial pressure, blood glucose concentration, and age. J Cereb Blood Flow Metab. 1988;8:449–461. doi: 10.1038/jcbfm.1988.86. [DOI] [PubMed] [Google Scholar]
- Coyle P. Different susceptibilities to cerebral infarction in spontaneously hypertensive (SHR) and normotensive Sprague-Dawley rats. Stroke. 1986;17:520–525. doi: 10.1161/01.str.17.3.520. [DOI] [PubMed] [Google Scholar]
- Slivka AP. Hypertension and hyperglycemia in experimental stroke. Brain Res. 1991;562:66–70. doi: 10.1016/0006-8993(91)91187-6. [DOI] [PubMed] [Google Scholar]
- Bouley J, Fisher M, Henninger N. Comparison between coated vs. uncoated suture middle cerebral artery occlusion in the rat as assessed by perfusion/diffusion weighted imaging. Neurosci Lett. 2007;412:185–190. doi: 10.1016/j.neulet.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Schmid-Elsaesser R, Zausinger S, Hungerhuber E, Baethmann A, Reulen HJ. A critical reevaluation of the intraluminal thread model of focal cerebral ischemia: evidence of inadvertent premature reperfusion and subarachnoid hemorrhage in rats by laser-Doppler flowmetry. Stroke. 1998;29:2162–2170. doi: 10.1161/01.str.29.10.2162. [DOI] [PubMed] [Google Scholar]
- Shimamura N, Matchett G, Tsubokawa T, Ohkuma H, Zhang J. Comparison of silicon-coated nylon suture to plain nylon suture in the rat middle cerebral artery occlusion model. J Neurosci Methods. 2006;156:161–165. doi: 10.1016/j.jneumeth.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Shigeno T, McCulloch J, Graham DI, Mendelow AD, Teasdale GM. Pure cortical ischemia versus striatal ischemia. Circulatory, metabolic, and neuropathologic consequences. Surgical neurology. 1985;24:47–51. doi: 10.1016/0090-3019(85)90063-1. [DOI] [PubMed] [Google Scholar]
- Bederson JB. Rat middle cerebral artery occlusion: evaluation of the model and development of a neurologic examination. Stroke. 1986;17:472–476. doi: 10.1161/01.str.17.3.472. [DOI] [PubMed] [Google Scholar]


