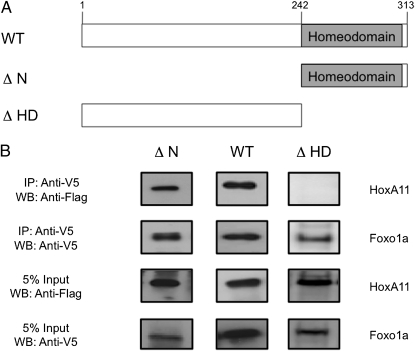
Fig. 3.
Homeodomain is sufficient to bind Foxo1a. (A) Cartoon of mouse HoxA11 deletion constructs used to determine the Foxo1a binding region. WT, full-length mouse HoxA11 (amino acids 1–313); ΔN, homeodomain plus 10 conserved amino acids (amino acids 242–313); ΔHD, homeodomain deleted (amino acids 1–241). (B) Deletion constructs were tested for their ability to coprecipitate with human Foxo1a. Flag epitope-tagged full-length HoxA11 (amino acids 1–313), homeodomain deleted (amino acids 1–241), and N-terminal deletion (amino acids 242–313) constructs were tested with V5/His-tagged Foxo1a. The presence of a WT and ΔN but no band for ΔHD after precipitation with Foxo1a, indicates that the C-terminal portion of the HoxA11 protein, which is composed of the homeodomain and 10 additional conserved amino acids, is sufficient to support the PPI with Foxo1a. Five percent input bands indicate equal expression of all constructs. Mammalian expression vectors were cotransfected into HeLa cells, and nuclear lysates were incubated with anti-V5 agarose (Sigma) overnight. The following day, samples were treated with DNaseI and washed, and protein complexes were eluted with NuPage LDS sample buffer. Protein complexes were resolved by SDS/PAGE and transferred to a PDVF membrane. Blots were probed with anti-Flag (1:100,000; Sigma) and anti-V5 (1:5,000; Invitrogen) antibodies. Experiments were repeated a minimum of three times, and results presented here are representative of typical findings. WB, Western blot.