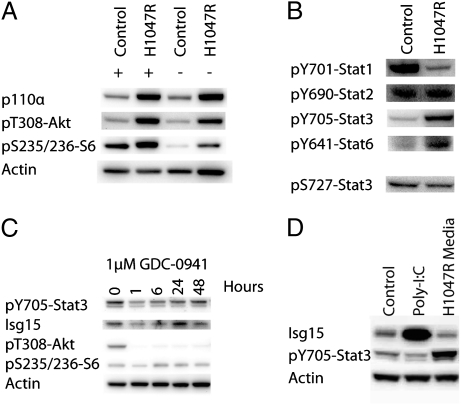
Fig. 1.
(A) PI3K signaling in cells transformed by the H1047R mutant of p110α. The Western blot shows expression of p110α and phosphorylation of Akt and of S6 in the presence and in the absence of serum. H1047R-transformed cells show constitutive activation of PI3K signaling in the absence of serum. (B) Phosphorylation of Stat proteins. 10T1/2 and 10T1/2-H1047R cells were lysed and probed for the indicated tyrosine phosphorylations of Stat proteins. Transformation by p110α-H1047R induces an increase in Stat3 and Stat6 phosphorylation and a decrease in Stat1 phosphorylation. S727 of Stat3, a known TOR phosphorylation site, is unaffected. (C) The PI3K inhibitor GDC-0941 blocks tyrosine phosphorylation of Stat3. The 10T1/2-H1047R cells were treated with 20 μM GDC-0941 for 0, 1, 6, 24, or 48 h as indicated and then analyzed by Western blot. (D) IFN stimulation. 10T1/2 cells were exposed to conditioned medium from either 10T1/2-H1047R or from normal 10T1/2 cells transfected with poly-I:C. The IFN produced by normal 10T1/2 cells in response to poly-I:C causes a sharp rise in the expression of Isg15 but does not induce phosphorylation of Stat3. In contrast, H1047R-conditioned medium leads to Stat3 phosphorylation but does not change Isg15 expression.