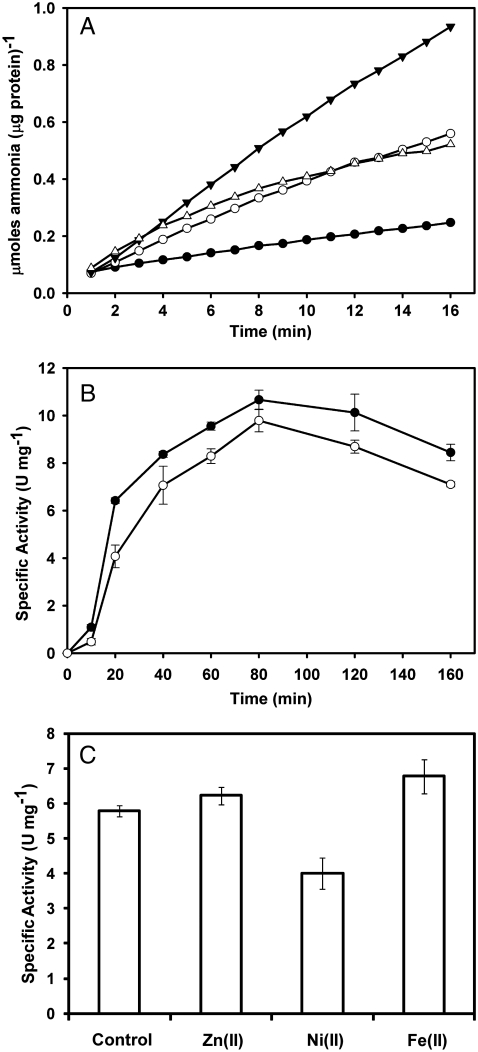
Fig. 1.
Activity of purified UreA2B2. (A) Progress curves for UreA2B2 purified anaerobically from H. mustelae when assayed in anaerobic buffer containing 50 mM urea (empty circle) or further supplemented with 3 mM acetohydroxamic acid (filled circles), 1 mM EDTA (solid upside down triangle), or 100 μM NiCl2 (empty triangle). Data points represent the average of duplicate experiments. (B) Activation kinetics of oxidized UreA2B2 by dithionite. Inactive samples (∼10 μM heterodimer) purified aerobically from H. mustelae (filled circles) and E. coli (open circles) were subjected to vacuum/argon cycling, supplemented with ∼100 μM dithionite, and incubated at ambient temperature. At the indicated time points, samples were assayed anaerobically in buffer containing 50 mM urea. Data points represent the average of triplicate experiments ± standard deviation. (C) Specific activity after reductive activation of E. coli-derived protein in the presence of metal ions. Protein (∼10 μM) was incubated with 1 mM dithionite and one equivalent of FeSO4, NiCl2, or ZnCl2 for 90 min before aliquots were assayed. Bars represent the average of triplicate experiments ± standard deviation.