Abstract
The transcription factor B-Myb plays a critical role in regulating gene expression and is implicated in controlling carcinogenesis and cellular senescence. Transcription of the B-Myb gene is regulated by retinoblastoma proteins acting directly on the B-Myb promoter. Recently, we found that microRNAs also control the abundance of B-Myb mRNA during senescence, adding another level of complexity to B-Myb regulation. This review focuses on the importance of B-Myb in cancer and senescence, with an emphasis on the regulation of B-Myb expression and activity.
Introduction
B-Myb (v-Myb myeloblastosis viral oncogene homolog [avian]-like 2 [MYBL2]) encodes a transcription factor that regulates the expression of numerous genes during cell cycle progression (1). The Myb gene family of transcription factors is present in all vertebrates (2). c-Myb is the homologue of the v-Myb oncogene, which is present in avian retroviruses that cause acute leukemia (3, 4). The other two family members, A-Myb and B-Myb, were cloned based on homology to c-Myb (5). In mammals, c-Myb and A-Myb expression is restricted to specific cell types and stages of development, whereas B-Myb is expressed in virtually all proliferating cells (2, 5). In cultured cells, B-Myb expression is highest in the S phase of the cell cycle, and changes in B-Myb expression have been linked to growth arrest, apoptosis, carcinogenesis, and senescence (6, 7). Thus, it is essential to determine the mechanisms that regulate expression of this important gene.
B-Myb regulates genes important in cell proliferation and survival
All three Myb proteins bind to consensus Myb-binding sites (MBS) in DNA (2), but the phenotypes of knockout mice that lack individual Myb family members are strikingly different, with mice lacking B-Myb dying very early in development because the blastocyst inner cell mass does not form (8). These diverse phenotypes demonstrate that the Myb proteins carryout different biological functions. Divergent function of the Myb proteins was also suggested by expression profiling, which revealed that ectopic expression of each of the three Myb proteins activates different sets of genes (9).
B-Myb stimulates transcription of genes that promote entry into the S and M phases of the cell cycle (1, 6). For several of these genes, such as DNA topoisomerase IIα and c-Myc, B-Myb acts by binding directly to MBSs in their promoters {e.g., (10, 11)}. B-Myb activates the expression of other genes that lack MBSs, including B-Myb itself, by interacting with transcription factors that bind to the promoters of these genes (12, 13). In conjunction with E2F proteins, B-Myb also stimulates the expression of genes required for the G2/M phase of the cell cycle (14). In addition, binding of B-Myb to the multiprotein LINC/DREAM complex regulates the ability of this complex to affect gene expression (15-18). B-Myb can also negatively repress gene expression, perhaps by competing with other transcription factors whose binding sites overlap with MBSs in target promoters {e.g., (19, 20)}. Finally, B-Myb associates directly with clathrin and filamin, components of the mitotic spindle (21). The absence of B-Myb reduces the amount of clathrin in the spindle and causes mitotic arrest.
The transcriptional activity of B-Myb is regulated by post-translational modifications and by interactions with other proteins (1). B-Myb is activated by phosphorylation and acetylation, which release it from transcriptional co-repressors (22, 23). However, phosphorylation also leads to ubiquitylation of the B-Myb protein (24), decreasing its half-life and limiting its activity to S phase. B-Myb activity is also regulated by binding to transcriptional co-activators, co-repressors, and other proteins {reviewed in (6)}.
B-Myb in cancer and senescence
Given the provenance of c-Myb as a cellular proto-oncogene and the ability of B-Myb to regulate the expression of cell cycle genes, it is not surprising that B-Myb is involved in cell proliferation and carcinogenesis. B-Myb expression is required for entry into S phase and can overcome growth inhibitory signals (25-27). Cytogenetic analysis of several types of cancers revealed amplification of chromosome 20q13, where B-Myb is located {e.g., (28)}. In addition, B-Myb over-expression occurs in several cancers and has been linked to aggressive tumor growth and poor outcomes in neuroblastomas and other tumors {e.g., (28-30)}. Conversely, B-Myb repression can inhibit the proliferation of normal and tumor cells (11, 26, 31, 32). The ability of B-Myb to increase the expression of anti-apoptotic genes such as Bcl2, survivin, and clusterin may also contribute to cancer progression (17, 33-35). Interestingly, certain inherited sequence variants of B-Myb are associated with altered cancer risk (30, 36).
Cellular senescence, a form of irreversible growth arrest, appears to be an important obstacle that cells must bypass during carcinogenesis (37). Because B-Myb expression is strongly repressed during senescence, it seems reasonable that loss of B-Myb expression may play an important role in senescence. Several findings support this hypothesis. Repression of B-Myb inhibits proliferation of mouse BALB/c3T3 fibroblasts, whereas constitutive B-Myb expression allows the cells to grow with reduced growth factors (32). In primary mouse embryonic fibroblasts, over-expression of the ras oncogene induces premature senescence, but co-expression of B-Myb abrogates this response (20). Our group previously showed that inhibition of B-Myb expression by shRNAs induces senescence in primary human fibroblasts and HeLa cervical cancer cells (38). In human embryonic lung fibroblasts, B-Myb is a direct transcriptional repressor of the cyclin-dependent kinase inhibitor p16INK4a, which is involved in the induction of senescence (39). Over-expression of B-Myb in these cells increases their in vitro lifespan, and B-Myb repression induces premature senescence. Taken together, these data suggest that B-Myb plays a central role in controlling senescence.
B-Myb is regulated at the post-transcriptional level by microRNAs during senescence
B-Myb expression is low in quiescent cells because of Rb-mediated repression (7). Repressive complexes between members of the E2F family and the Rb family (specifically E2F4/p107 and E2F4/p130) bind to E2F sites in the B-Myb promoter in G0 cells and repress its activity (40). Recently, our group discovered that B-Myb is also repressed at the post-transcriptional level by microRNAs (41).
Small non-coding microRNAs regulate gene expression by base-pairing with specific mRNA targets and affecting their translation or stability. We found that the expression of approximately 50 cellular microRNAs changes in HeLa cells undergoing Rb-induced senescence. Several members of the miR-29 and miR-30 families are up-regulated in senescent HeLa cells and primary human foreskin fibroblasts. This up-regulation is Rb-dependent and likely to involve the oncogene c-Myc. It has been shown that c-Myc binds directly to the miR-29 and miR-30 promoters and acts as a transcriptional repressor of these genes (42). However, c-Myc itself is an E2F-responsive gene repressed by the Rb-pathway during senescence (43). Taken together, these data suggest that miR-29 and miR-30 up-regulation during senescence is due to Rb-driven inhibition of c-Myc expression and the consequent loss of c-Myc-mediated repression of the promoters controlling transcription of these microRNAs.
By using reporter constructs, mutational analysis of microRNA binding sites, and ectopic expression or inhibition of members of the miR-29 and miR-30 families in both cell types, we demonstrated that these microRNAs bind to the 3′UTR of B-Myb mRNA during senescence and reduce the amount of this mRNA, presumably by reducing its stability. Furthermore, overexpression of miR-29 and miR-30 inhibits the expression of endogenous B-Myb and reduces DNA synthesis. The inhibition of DNA synthesis by these microRNAs was partially rescued by exogenous expression of B-Myb, suggesting that B-Myb repression is responsible, at least in part, for growth inhibition in cells over-expressing miR-29 and miR-30. Finally, antagonizing the activity of miR-29 and miR-30 allows a population of cells to escape Rb-induced senescence, demonstrating the importance of miR-29 and miR-30 in this process.
These experiments demonstrate the complexity of B-Myb regulation during Rb-induced senescence (Figure 1). Activation of the Rb pathway mobilizes p107 and p130 which, in complex with E2F4, bind to the B-Myb promoter and inhibit transcription. In addition, Rb activation increases the expression of miR-29 and miR-30, which bind to B-Myb mRNA and destabilize it. As noted above, B-Myb is also regulated at the protein level by post-translational modification and association with other cellular proteins. Presumably, this three-tiered regulation of B-Myb evolved to ensure profound inhibition of B-Myb activity and complete blockade of cell cycle progression.
Figure 1. Three-tiered control of B-Myb.
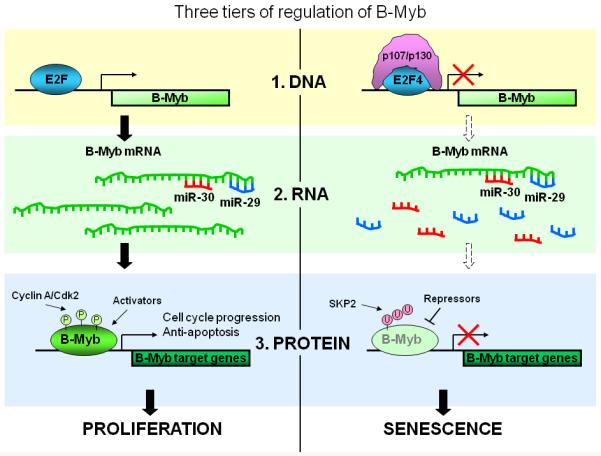
Activation of the Rb pathway during senescence restricts B-sMyb action at multiple levels. At the DNA level, activating E2F complexes at the B-Myb promoter are replaced by inhibitory complexes between E2F4 and p107 and p130. At the RNA level, elevated levels of miR-29 and miR-30 base-pair to the 3′UTR of B-Myb mRNA, reducing its abundance. At the protein level, reduced expression of B-Myb-responsive genes lowers cyclin/cdk activity and the extent of activating phosphorylation of B-Myb. In addition, ubiquitylation and degradation of B-Myb, as well as preferential association with co-repressors, may further inhibit its ability to activate target genes.
miR-29 and miR-30 affect the expression of numerous genes in addition to B-Myb, and many of these targets may also play a role in senescence or carcinogenesis. For example, Croce and colleagues have identified DNA methyltransferase 3A and 3B as targets of miR-29 (44). Similarly, in addition to miR-29 and miR-30, numerous microRNAs are up-regulated or down-regulated during senescence (41). These microRNAs may also regulate the expression of genes involved in senescence.
Conclusions and perspectives
B-Myb exerts powerful effects on cell behavior. The multitude of B-Myb protein partners and target genes allows it to regulate many important cellular processes including proliferation, senescence, apoptosis, and mitosis. Given this spectrum of activities and its association with human cancer, it may be possible to exploit B-Myb as an important prognostic, diagnostic and therapeutic tool. Do mutations that affect the binding of Rb proteins to the B-Myb promoter or the binding of microRNAs to B-Myb mRNA affect cancer risk by regulating levels of B-Myb? Is the presence of such mutations or the level of B-Myb itself an informative biomarker? Is it possible to influence the activity of B-Myb and effect cellular behavior by modulating its expression or its ability to bind to DNA or its protein partners, including components of the LINC/DREAM complex and the mitotic spindle? Are there as-yet-unrecognized layers of complexity in the regulation of B-Myb that can be exploited therapeutically? The central role played by B-Myb in cellular proliferation and related processes suggests that the factors that regulate the expression and activity of B-Myb deserve special scrutiny in attempts to develop rational approaches to control cancer.
Acknowledgments
We thank Joan Steitz and members of the DiMaio laboratory for critical reading of the manuscript.
Grant support
Work conducted in the authors’ laboratory was supported by a grant from the National Cancer Institute (CA016038).
Footnotes
There are no potential conflicts of interest.
References
- 1.Joaquin M, Watson RJ. Cell cycle regulation by the B-Myb transcription factor. Cell Mol Life Sci. 2003;60:2389–401. doi: 10.1007/s00018-003-3037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oh IH, Reddy EP. The myb gene family in cell growth, differentiation and apoptosis. Oncogene. 1999;18:3017–33. doi: 10.1038/sj.onc.1202839. [DOI] [PubMed] [Google Scholar]
- 3.Roussel M, Saule S, Lagrou C, Rommens C, Beug H, Graf T, et al. Three new types of viral oncogene of cellular origin specific for haematopoietic cell transformation. Nature. 1979;281:452–5. doi: 10.1038/281452a0. [DOI] [PubMed] [Google Scholar]
- 4.Shen-Ong GL. The myb oncogene. Biochim Biophys Acta. 1990;1032:39–52. doi: 10.1016/0304-419x(90)90011-o. [DOI] [PubMed] [Google Scholar]
- 5.Nomura N, Takahashi M, Matsui M, Ishii S, Date T, Sasamoto S, et al. Isolation of human cDNA clones of myb-related genes, A-myb and B-myb. Nucleic Acids Res. 1988;16:11075–89. doi: 10.1093/nar/16.23.11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sala A. B-MYB, a transcription factor implicated in regulating cell cycle, apoptosis and cancer. Eur J Cancer. 2005;41:2479–84. doi: 10.1016/j.ejca.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Lam EW, Bennett JD, Watson RJ. Cell-cycle regulation of human B-myb transcription. Gene. 1995;160:277–81. doi: 10.1016/0378-1119(95)00184-8. [DOI] [PubMed] [Google Scholar]
- 8.Tanaka Y, Patestos NP, Maekawa T, Ishii S. B-myb is required for inner cell mass formation at an early stage of development. J Biol Chem. 1999;274:28067–70. doi: 10.1074/jbc.274.40.28067. [DOI] [PubMed] [Google Scholar]
- 9.Ness SA. Myb protein specificity: evidence of a context-specific transcription factor code. Blood Cells Mol Dis. 2003;31:192–200. doi: 10.1016/s1079-9796(03)00151-7. [DOI] [PubMed] [Google Scholar]
- 10.Brandt TL, Fraser DJ, Leal S, Halandras PM, Kroll AR, Kroll DJ. c-Myb trans-activates the human DNA topoisomerase II alpha gene promoter. J Biol Chem. 1997;272:6278–84. doi: 10.1074/jbc.272.10.6278. [DOI] [PubMed] [Google Scholar]
- 11.Gualdrini F, Corvetta D, Cantilena S, Chayka O, Tanno B, Raschella G, et al. Addiction of MYCN amplified tumours to B-MYB underscores a reciprocal regulatory loop. Oncotarget. 2010;1:278–88. doi: 10.18632/oncotarget.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foos G, Natour S, Klempnauer KH. TATA-box dependent trans-activation of the human HSP70 promoter by Myb proteins. Oncogene. 1993;8:1775–82. [PubMed] [Google Scholar]
- 13.Sala A, Saitta B, De Luca P, Cervellera MN, Casella I, Lewis RE, et al. B-MYB transactivates its own promoter through SP1-binding sites. Oncogene. 1999;18:1333–9. doi: 10.1038/sj.onc.1202421. [DOI] [PubMed] [Google Scholar]
- 14.Zhu W, Giangrande PH, Nevins JR. E2Fs link the control of G1/S and G2/M transcription. EMBO J. 2004;23:4615–26. doi: 10.1038/sj.emboj.7600459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Litovchick L, Sadasivam S, Florens L, Zhu X, Swanson SK, Velmurugan S, et al. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol Cell. 2007;26:539–51. doi: 10.1016/j.molcel.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 16.Schmit F, Korenjak M, Mannefeld M, Schmitt K, Franke C, von Eyss B, et al. LINC, a human complex that is related to pRB-containing complexes in invertebrates regulates the expression of G2/M genes. Cell Cycle. 2007;6:1903–13. doi: 10.4161/cc.6.15.4512. [DOI] [PubMed] [Google Scholar]
- 17.Knight AS, Notaridou M, Watson RJ. A Lin-9 complex is recruited by B-Myb to activate transcription of G2/M genes in undifferentiated embryonal carcinoma cells. Oncogene. 2009;28:1737–47. doi: 10.1038/onc.2009.22. [DOI] [PubMed] [Google Scholar]
- 18.Mannefeld M, Klassen E, Gaubatz S. B-MYB is required for recovery from the DNA damage-induced G2 checkpoint in p53 mutant cells. Cancer Res. 2009;69:4073–80. doi: 10.1158/0008-5472.CAN-08-4156. [DOI] [PubMed] [Google Scholar]
- 19.Marhamati DJ, Sonenshein GE. B-Myb expression in vascular smooth muscle cells occurs in a cell cycle-dependent fashion and down-regulates promoter activity of type I collagen genes. J Biol Chem. 1996;271:3359–65. doi: 10.1074/jbc.271.7.3359. [DOI] [PubMed] [Google Scholar]
- 20.Masselink H, Vastenhouw N, Bernards R. B-myb rescues ras-induced premature senescence, which requires its transactivation domain. Cancer Lett. 2001;171:87–101. doi: 10.1016/s0304-3835(01)00631-0. [DOI] [PubMed] [Google Scholar]
- 21.Yamauchi T, Ishidao T, Nomura T, Shinagawa T, Tanaka Y, Yonemura S, et al. A B-Myb complex containing clathrin and filamin is required for mitotic spindle function. EMBO J. 2008;27:1852–62. doi: 10.1038/emboj.2008.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson LR, Johnson TK, Desler M, Luster TA, Nowling T, Lewis RE, et al. Effects of B-Myb on gene transcription: phosphorylation-dependent activity and acetylation by p300. J Biol Chem. 2002;277:4088–97. doi: 10.1074/jbc.M105112200. [DOI] [PubMed] [Google Scholar]
- 23.Robinson C, Light Y, Groves R, Mann D, Marias R, Watson R. Cell-cycle regulation of B-Myb protein expression: specific phosphorylation during the S phase of the cell cycle. Oncogene. 1996;12:1855–64. [PubMed] [Google Scholar]
- 24.Charrasse S, Carena I, Brondani V, Klempnauer KH, Ferrari S. Degradation of B-Myb by ubiquitin-mediated proteolysis: involvement of the Cdc34-SCF(p45Skp2) pathway. Oncogene. 2000;19:2986–95. doi: 10.1038/sj.onc.1203618. [DOI] [PubMed] [Google Scholar]
- 25.Lin D, Fiscella M, O’Connor PM, Jackman J, Chen M, Luo LL, et al. Constitutive expression of B-myb can bypass p53-induced Waf1/Cip1-mediated G1 arrest. Proc Natl Acad Sci U S A. 1994;91:10079–83. doi: 10.1073/pnas.91.21.10079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sala A, Casella I, Bellon T, Calabretta B, Watson RJ, Peschle C. B-myb promotes S phase and is a downstream target of the negative regulator p107 in human cells. J Biol Chem. 1996;271:9363–7. doi: 10.1074/jbc.271.16.9363. [DOI] [PubMed] [Google Scholar]
- 27.Joaquin M, Watson RJ. The cell cycle-regulated B-Myb transcription factor overcomes cyclin-dependent kinase inhibitory activity of p57(KIP2) by interacting with its cyclin-binding domain. J Biol Chem. 2003;278:44255–64. doi: 10.1074/jbc.M308953200. [DOI] [PubMed] [Google Scholar]
- 28.Bar-Shira A, Pinthus JH, Rozovsky U, Goldstein M, Sellers WR, Yaron Y, et al. Multiple genes in human 20q13 chromosomal region are involved in an advanced prostate cancer xenograft. Cancer Res. 2002;62:6803–7. [PubMed] [Google Scholar]
- 29.Raschella G, Cesi V, Amendola R, Negroni A, Tanno B, Altavista P, et al. Expression of B-myb in neuroblastoma tumors is a poor prognostic factor independent from MYCN amplification. Cancer Res. 1999;59:3365–8. [PubMed] [Google Scholar]
- 30.Thorner AR, Hoadley KA, Parker JS, Winkel S, Millikan RC, Perou CM. In vitro and in vivo analysis of B-Myb in basal-like breast cancer. Oncogene. 2009;28:742–51. doi: 10.1038/onc.2008.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Arsura M, Introna M, Passerini F, Mantovani A, Golay J. B-myb antisense oligonucleotides inhibit proliferation of human hematopoietic cell lines. Blood. 1992;79:2708–16. [PubMed] [Google Scholar]
- 32.Sala A, Calabretta B. Regulation of BALB/c 3T3 fibroblast proliferation by B-myb is accompanied by selective activation of cdc2 and cyclin D1 expression. Proc Natl Acad Sci U S A. 1992;89:10415–9. doi: 10.1073/pnas.89.21.10415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cervellera M, Raschella G, Santilli G, Tanno B, Ventura A, Mancini C, et al. Direct transactivation of the anti-apoptotic gene apolipoprotein J (clusterin) by B-MYB. J Biol Chem. 2000;275:21055–60. doi: 10.1074/jbc.M002055200. [DOI] [PubMed] [Google Scholar]
- 34.Grassilli E, Salomoni P, Perrotti D, Franceschi C, Calabretta B. Resistance to apoptosis in CTLL-2 cells overexpressing B-Myb is associated with B-Myb-dependent bcl-2 induction. Cancer Res. 1999;59:2451–6. [PubMed] [Google Scholar]
- 35.Lang G, Gombert WM, Gould HJ. A transcriptional regulatory element in the coding sequence of the human Bcl-2 gene. Immunology. 2005;114:25–36. doi: 10.1111/j.1365-2567.2004.02073.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwab R, Bussolari R, Corvetta D, Chayka O, Santilli G, Kwok JM, et al. Isolation and functional assessment of common, polymorphic variants of the B-MYB proto-oncogene associated with a reduced cancer risk. Oncogene. 2008;27:2929–33. doi: 10.1038/sj.onc.1210947. [DOI] [PubMed] [Google Scholar]
- 37.Ben-Porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005;37:961–76. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 38.Johung K, Goodwin EC, DiMaio D. Human papillomavirus E7 repression in cervical carcinoma cells initiates a transcriptional cascade driven by the retinoblastoma family, resulting in senescence. J Virol. 2007;81:2102–16. doi: 10.1128/JVI.02348-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huang Y, Wu J, Li R, Wang P, Han L, Zhang Z, et al. B-MYB delays cell aging by repressing p16 (INK4alpha) transcription. Cell Mol Life Sci. 2011;68:893–901. doi: 10.1007/s00018-010-0501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells J, Boyd KE, Fry CJ, Bartley SM, Farnham PJ. Target gene specificity of E2F and pocket protein family members in living cells. Mol Cell Biol. 2000;20:5797–807. doi: 10.1128/mcb.20.16.5797-5807.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez I, Cazalla D, Almstead LL, Steitz JA, DiMaio D. miR-29 and miR-30 regulate B-Myb expression during cellular senescence. Proc Natl Acad Sci U S A. 2011;108:522–7. doi: 10.1073/pnas.1017346108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, et al. Widespread microRNA repression by Myc contributes to tumorigenesis. Nat Genet. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.DeFilippis RA, Goodwin EC, Wu L, DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Virol. 2003;77:1551–63. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, et al. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–10. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]


