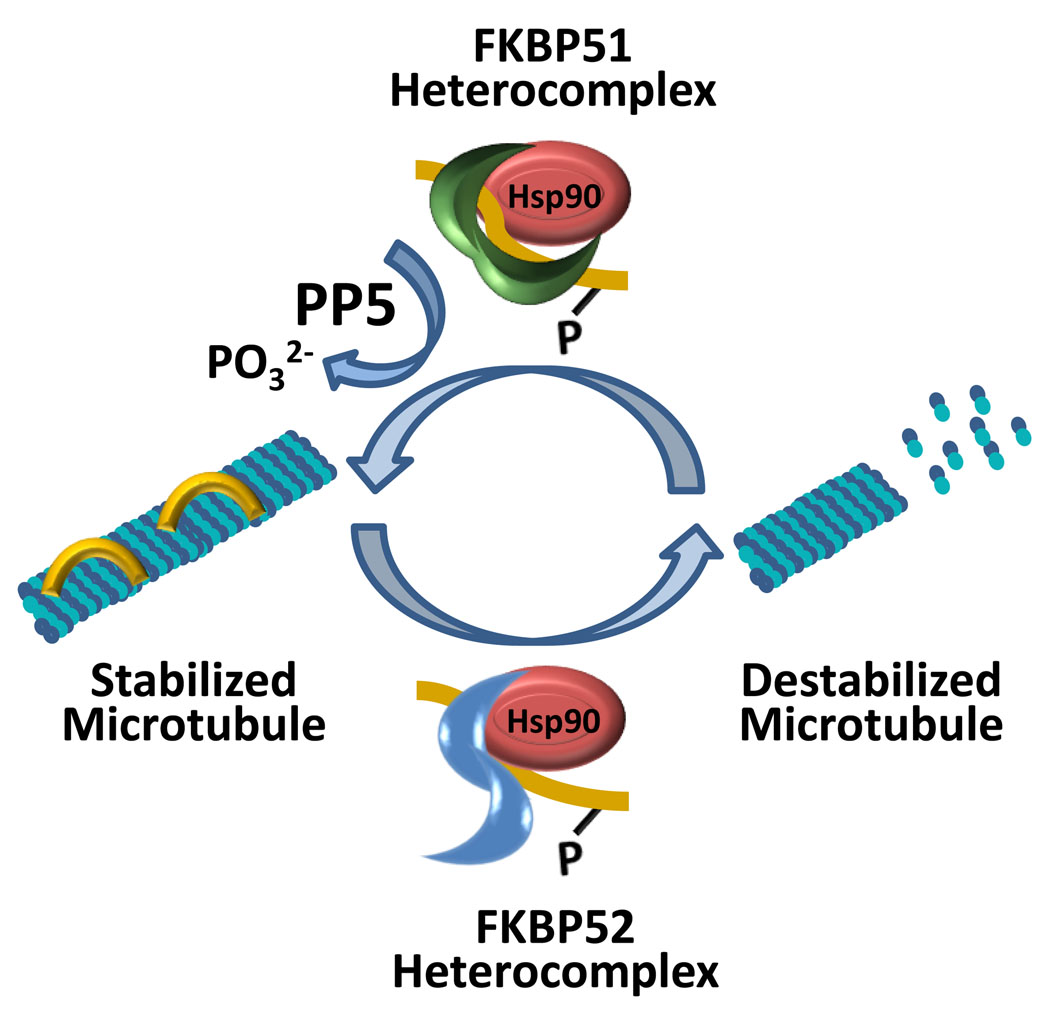
Figure 3.
Model of FKBP51- and FKBP52-mediated regulation of microtubule dynamics. Microtubules are stabilized by the binding of cis tau, but are destabilized when tau becomes phosphorylated and can no longer bind. Phosphorylated tau in a trans configuration can be part of an FKBP51 heterocomplex or an FKBP52 heterocomplex. These heterocomplexes consist of at least FKBP51/FKBP52, phosphorylated trans tau, and Hsp90. In the FKBP51 heterocomplex, the TPR domain of FKBP51 (green) mediates interaction with Hsp90, and its FK1 domain is spatially oriented towards phosphorylated trans tau (yellow). In this orientation, FKBP51 catalyzes isomerization to the cis configuration that allows the phosphatase PP5 access to tau. PP5 dephosphorylates cis tau, which recycles to bind to and stabilize microtubules. In the FKBP52 heterocomplex, while the TPR domain of FKBP52 (blue) mediates binding to Hsp90, its FK1 domain is oriented away from phosphorylated trans tau thereby preventing PPIase activity on tau. Tau remains in a phosphorylated trans configuration unable to bind to microtubules, which leads to microtubule destabilization.