Abstract
Social evolution in honey bees has produced strong queen-worker dimorphism for plastic traits that depend on larval nutrition. The honey bee developmental program includes both larval components that determine plastic growth responses to larval nutrition and nurse components that regulate larval nutrition. We studied how these two components contribute to variation in worker and queen body size and ovary size for two pairs of honey bee lineages that show similar differences in worker body-ovary size allometry but have diverged over different evolutionary time scales. Our results indicate: that the lineages have diverged for both nurse and larval developmental components, that rapid changes in worker body-ovary allometry may disrupt queen development, and that queen-worker dimorphism arises mainly from discrete nurse-provided nutritional environments, not from a developmental switch that converts variable nutritional environments into discrete phenotypes. Both larval and nurse components have likely contributed to the evolution of queen-worker dimorphism.
Keywords: allometry, eusociality, indirect genetic effects, interacting phenotypes, developmental evolution
Introduction
Ancestrally, bees are solitary and females are monomorphic for traits such as body size and ovary size (Michener, 2000). In contrast, in some highly derived social lineages, such as the honey bees (genus Apis), there is a reproductive division of labor characterized by strong queen-worker dimorphism for plastic traits such as body size and especially ovary size that depend on nutrition (Hölldobler & Wilson, 1990). Honey bee queens are approximately twice as large as workers in terms of mass, but queens can have more than 360 total ovarioles while workers typically have fewer than ten (Michener, 1974, Winston, 1987). Large queen ovaries enable a high egg-laying rate (more than 1500 per day) and are apparently an adaptation for large colony size and colony reproduction by fission (Winston, 1987). Thus, social evolution in bees leading to the elaboration of queen-worker dimorphism has involved dramatic changes in plasticity for body size and ovary size and the expressed allometric body-ovary size relationship.
Queen-worker caste dimorphism has often been regarded as a prime example of a polyphenism, environmentally-induced alternative phenotypes (Wheeler, 1986). In honey bees, it has long been known that the expression of queen versus worker traits depends mainly on larval nutrition (Weaver, 1955, Wheeler, 1986). However, evidence has accumulated in other social insect lineages that genotype also influence the expression of alternative caste phenotypes (Schwander et al. 2010, Anderson et al., 2008), and even in honey bees there is evidence of genetic influences on the expression of caste and caste-related traits (e.g., Allsopp et al., 2003, Beekman et al., 2000, Osborne & Oldroyd, 1999, Linksvayer et al., 2009b). The genetic modification of pre-exisiting developmental plasticity is thought to contribute to the evolution of polyphenisms (Nijhout, 2003, Moczek & Nijhout, 2002, Emlen et al., 2007), and studying patterns of genetic variation for developmental plasticity and the expression of caste-related traits may provide insight into the evolution of queen-worker dimorphism.
While previous studies of the developmental genetic basis of honey bee queen-worker dimorphism have focused solely on larval genes involved in plastic developmental responses (Evans & Wheeler, 1999, Wheeler et al., 2006, Barchuk et al., 2007, Patel et al., 2007, de Azevedo & Hartfelder, 2008), whether a larva develops into a queen or worker can also depend on genes expressed in nestmates that actively regulate the larval environment (Linksvayer & Wade, 2005). In particular, nurse workers constrain the range of nutritional environments experienced by larvae and thereby control the expression of phenotypic plasticity. Thus the social insect developmental program potentially has two distinct genetic components that are expected to co-evolve to shape expressed phenotypes (Wolf & Brodie, 1998, Linksvayer & Wade, 2005, Linksvayer, 2006, Linksvayer, 2007, Linksvayer et al., 2009a). Indeed a range of studies provide evidence that social insect traits, including caste, are influenced by interactions between the genotypes of developing larvae and the genotypes of nurse workers (e.g., Allsopp et al., 2003, Jarau et al., 2010, Beekman et al., 2000, Osborne & Oldroyd, 1999, Rinderer et al., 1986, Linksvayer et al., 2009a, Linksvayer et al., 2009b, Pankiw et al., 2002). These studies suggest that approaches considering the contribution of social regulation of development can complement previous studies focused on differential gene expression in queen- and worker-destined larvae.
Here we study the contribution of larval and nurse components of the developmental program to variation between two pairs of honey bee lineages for the relationship between body size and ovary size in queens and workers. Our two pairs of study lineages are Africanized honey bees (AHB) from a population in south-central Arizona and European honey bees (EHB) from commercial U.S. stocks, and the selected high and low pollen hoarding strains of Page and Fondrk (1995) derived from commercial European U.S. stocks. The two pairs of divergent lineages show similar differences in worker body size and ovary size but have diverged over very different evolutionary time scales: AHB and EHB are derived from lineages that initially diverged about 1 million years ago (Whitfield et al., 2006), and the high and low pollen hoarding strains have been produced by 33 generations of artificial selection over 20 years, for a colony-level trait, the amount of pollen stored in the colony. As a result of the artificial selection program, high pollen hoarding worker bees are on average smaller in body size but have larger ovaries (more ovarioles), and similarly, AHB are smaller but with larger ovaries than EHB (Amdam et al., 2006, Linksvayer et al., 2009b). We use in vivo cross-fostering to determine how changes in regulation of the social environment contribute to differences between lineages; then we use in vitro rearing to study how the bee lineages respond to variation in the nutritional environment, independent of social regulation. Our results can provide insight into the evolution of developmental plasticity for body size and ovary size that contributes to the expression of queen-worker dimorphism.
Materials and methods
Colony sources
Eight colony sources were used: two AHB colonies established from swarms collected near Mesa, Arizona, two EHB colonies from commercial stocks, and two colonies each from the high and low pollen hoarding strains of Page and Fondrk (1995).
Colony-reared workers and queens
We created four pairs of colonies matched for size, two pairs consisting of a matched AHB colony and a EHB colony, and two pairs consisting of a matched high pollen hoarding strain colony and a low pollen hoarding strain colony. Cross-fostering was carried out between colonies within each of these pairs (i.e. between AHB and EHB and between high and low pollen hoarding strains), first to produce colony-reared workers and then colony-reared queens.
Queens of all genotypes (i.e. lineages and strains) were caged for approximately 24 hours on frames matched for age and then three days later the frames with approximately 3 day old eggs were cross-fostered into one rearing environment for each pair of matched genotypes (e.g., frames with eggs from EHB colony 1 and AHB colony 1 were fostered to EHB colony 1). The two frames were placed in the colony facing each other to minimize within-colony environmental differences experienced by brood on the two cross-fostered frames. Frames with colony-reared workers were removed from the hive 24 hours before the workers began to emerge and placed in incubators. Any cells full of pollen or honey were covered by wax or foil so that newly emerged workers did not have access to food. Queens were then re-caged and three days later the frames cross-fostered into the alternate rearing environment (e.g., frames from EHB colony 1 and AHB colony 1 were fostered to AHB colony 1). 50 newly emerged workers were collected and phenotyped (see below) for each combination of colony source and colony rearing environment.
After producing colony-reared workers, colonies were prepared to produce queens. Queens were caged for approximately 24 hours. Then, to facilitate queen production (Laidlaw & Page, 1997), colonies were supplemented with saturated sugar syrup solution on the third day and on the fourth day queens were removed from the brood-rearing area of the colony. 15 larvae that were approximately 1 day old were grafted from each paired source into queen cell cups placed into each colony on frames as above. Frames with queen cells were removed from the hive 24 hours prior to emergence and placed in incubators. We monitored the cells every 6 hours and removed newly emerged queens for immediate weighing and subsequent ovary phenotyping (see below). The whole procedure was then repeated a second time so that in total 30 larvae were grafted into queen cells cups and reared to adulthood for each combination of colony source and colony rearing environment.
In vitro rearing
Individual larvae were reared in vitro in climate control chambers (34 C, approximately 85 % RH during larval stages and 70% RH during the pupal stages) following the protocol of Kaftanoglu et al. (Kaftanoglu et al., 2010) in which each grafted larva was fed a single time. Approximately 24 hour old larvae (obtained after caging queens and checking larvae under a microscope to ensure they were approximately the same size across colonies and treatments) were grafted directly onto the surface of their food, kept in cells made from the conical bottoms of 50 ml graduated plastic centrifuge tubes (Corning). Larvae in the ‘worker diet’ treatment were fed 175 mg of liquid diet and larvae in the ‘queen diet’ were fed 250 mg of liquid diet; according to preliminary trials these two quantities produced mainly normal-size workers and normal-sized queens, respectively. A total of 96 larvae per colony per treatment were grafted (i.e. for a grand total of 4 genotypes * 2 replicate colonies * 2 treatments * 96 larvae = 1536 larvae).
Cells were monitored daily and were removed as soon as the larva or pupa became discolored or was drowned in food, or if there was any evidence of fungal growth. Mortality occurred mainly during the first day after grafting and during pupation (apparently usually due to drowning in food and due to fungal infection, respectively). Larvae that survived to the pupal stage generally consumed all the food in both treatments and bees that survived pupation generally appeared to be healthy adults, with some individuals morphologically indistinguishable from colony-reared workers and queens.
Phenotyping
Newly emerged colony-reared workers were weighed to the nearest 0.1 mg using a Mettler-Toledo AB204-5 microbalance and both ovaries dissected and counted under a stereo microscope (after Linksvayer et al. 2009a,b). Newly emerged colony-reared queens and in vitro reared females were weighed, ovariole numbers counted, and caste-defining external traits (degree of mandibular notch and corbicula) scored on a discrete 0,1,2,3 scale. Individuals with only external worker morphological characters were dissected and ovariole number counted as for colony-reared workers. For counting the ovarioles of the remaining individuals that had at least some queen-like characters, we chose a more labor-intensive protocol because it is difficult to count ovariole number accurately for large queen-like ovaries with simple dissection. Briefly, specimens were fixed in Alcoholic Bouin's fixative for at least one week (Presnell & Martin, 1997); after fixation, the abdomens of specimens were removed, washed in 95% ethanol, dehydrated in 95% n-butanol and 100% n-butanol, cleared in xylene, and infiltrated with and embedded in Fisherbrand Paraplast X-tra tissue embedding medium; next abdomens were sectioned on a Surgipath Rotary microtome at 5 micrometers, stained with Gill's Hematoxylin Solution and counterstained with Eosin Solution, and mounted on gelatin coated microscope slides (Presnell & Martin, 1997); finally, ovariole counts were made by counting the number of individual cross-sectioned ovarioles with an American Optical compound microscope. Ovariole counts were made blindly with respect to genotype and treatment, and further a qualitative ovary quality score (0, 1, 2, or 3) was recorded for each count based on the clarity of individual ovarioles on the slide. Ovariole counts with the lowest quality score (0) were excluded from subsequent analysis to minimize downward bias in ovariole counts due to low quality sectioning or staining.
In order to categorize in vitro individuals further as being “worker-like”, “queen-like”, or “intercastes”, we converted ovariole counts into a discrete ovary size score from 0-3 where normal colony-reared workers would have a score of 0 and normal colony-reared queens would have a score of 3: 0 (ovariole number ≤ 50), 1 (ovariole number 50-85), 2 (ovariole number 86-124) or 3 (ovariole number ≥ 125). We added these ovary size scores to the scores for mandibular notch and corbicula to get a total “queenliness” score. Individuals with values in the range of colony-reared workers (0-1) were defined as “worker-like”, individuals with values in the range of colony-reared queens (7-9) were defined as “queen-like”, and individuals with values in between colony-reared workers and queens (2-6) were defined as “intercastes”.
Statistical analysis
All analyses were carried out in R version 2.10.1. Generalized linear mixed models (GLMM, using glmer and glmmPQL packages) with quasi-Poisson errors were used for worker ovariole number and general linear mixed models (GLM, using the lme package) were used for worker and queen mass and queen ovariole number. Larval genotype (strain or lineage) and rearing environment were included as fixed factors and colony replicate was included as a random factor (Linksvayer et al., 2009a, Linksvayer, 2007) .
Results
Colony-reared high and low pollen hoarding strain workers
The mass of focal individuals depended on their genotype (GLM, F1,396 = 8.21, p = 0.0044) and the rearing environment (GLM, F1,396 = 4.51, p = 0.034). Confirming the results of a previous study (Linksvayer et al., 2009a), we found that the ovariole number of high and low strain workers depends on an interaction between their own genotype and the rearing environment (see the electronic supplementary material, figure S1): focal individual genotype main effect (GLMM, n = 391, z = -6.64, p < 0.001; on average low strain individuals had 2.97 fewer ovarioles than high strain individuals), rearing environment main effect (GLMM, z = 7.71, p < 0.001; on average focal individuals reared by low strain nurses had 4.03 more ovarioles than when reared by high strain nurses), genotype x rearing environment interaction (GLMM, z = -3.76, p < 0.001; on average low strain focal individuals reared by low strain nurses had 2.35 fewer ovarioles than expected).
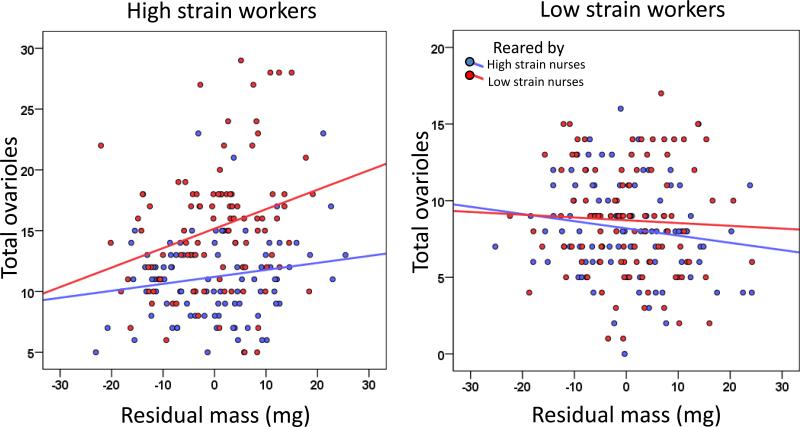
To investigate how combinations of focal individual genotype and rearing environment affects the relationship between body size and ovary size, we first used a full model for ovariole number including focal worker genotype, rearing environment, focal worker mass, all interactions, as well as replicate. The full model indicated complex interactions between genotype, rearing environment, and mass: there was a genotype x rearing environment effect (GLMM, n = 391, z = 1.989, p = 0.0467), but there were also rearing environment x mass (GLMM, z = 2.433, p = 0.0150) and genotype x rearing environment x mass effects (GLMM, z = -2.33, p = 0.0199) (figure 1). These results show that the relationship between worker body size and worker ovary size is conditional on worker genotype and the social environment. To disentangle these complex interactions we looked separately within each genotype of focal individuals.
Fig. 1.
Body-ovary size relationship for cross-fostered high and low pollen hoarding workers. High strain workers (left panel) and low strain workers (right panel) were reared to adulthood in either a high (blue) or low (red) strain social environment. Residual mass is plotted after controlling for differences between replicates colonies.
Within high strain focal workers, the relationship between mass and ovariole number was conditional on rearing environment (GLMM, rearing environment x mass, n = 193, z = 2.551, p = 0.0107), indicating that high strain larvae reared in different social environments had a different relationship between mass and ovariole number (figure 1). In contrast, for low strain focal workers, no factors predicted ovariole number even after removing the non-significant rearing environment x mass interaction (GLMM, n = 193, rearing environment, z = 1.69, p = 0.0916; mass, z = -1.53, p = 0.126), indicating that for low strain workers, there was no significant relationship between body size and ovary size in either rearing environment (figure 1). A model with replicate and worker mass showed that adult worker mass was positively associated with adult worker ovariole number when high strain larvae were reared in a low strain social environment (GLMM, n = 98, z = 5.436, p < 0.001), but there was no significant relationship for all other combinations of focal worker genotype and rearing environment (all p > 0.05).
Colony-reared AHB and EHB workers
The mass of AHB and EHB workers depended on their own genotype (GLM, F1,465 = 85.04, p < 0.0001) and the interaction between genotype and rearing environment (GLM, F1,465 = 8.67, p =0.0034); on average EHB workers were larger (effect = 12.80 mg, s.e. = 1.52, t=8.43), and the differences between AHB and EHB workers were less extreme in the EHB social environment (effect = -6.09 mg, s.e. = 2.07, t = -2.95)(see the electronic supplementary material, figure S2). Similarly to the results above, the interaction means that worker body size differences between AHB and EHB depends on both worker genotype and the genotypic composition of the social environment. Total ovariole number of AHB and EHB workers depended only on their own genotype; AHB workers on average had 5.15 more ovarioles than EHB workers (GLMM, s.e. = 0.0477, df = 445, t = -10.42, p < 0.0001) (see the electronic supplementary material, figure S2). There were no additional significant factors when mass and all two and three way interactions with genotype and rearing environment were added to the model. When AHB and EHB focal workers were examined separately, there was no significant interaction between rearing environment and mass (GLMM, p > 0.05), however both showed the same pattern (see the electronic supplementary material, figure S3), and when AHB and EHB focal individuals were pooled, there was a significant interaction between rearing environment and mass (GLMM, s.e. = 0.00423, df = 443, t = 2.25, p = 0.025). These results suggest that the mass-ovariole number relationship expressed by focal individuals depends on whether they were reared by AHB or EHB nurses.
Colony-reared high and low pollen hoarding strain queens
For queen mass, there was a main effect of genotype (GLM, F1,99 = 21.84, p < 0.0001; effect = 22.3, s.e. = 4.81, t = 4.64, see the electronic supplementary material, figure S4). There were no genotype or social rearing environment effects on total ovariole number (GLM, n = 103, all p > 0.05) for high and low strains queens. When mass and the additional interactions were added to the model predicting ovariole number, there was a positive relationship between mass and ovariole number (GLM, F1,98 = 7.14, p = 0.0088), with on average an increase of 0.442 ovarioles for every mg in mass (s.e. = 0.165, t = 2.67). When examining high and low genotype queens separately, there was no relationship between ovariole number and mass or rearing environment in low strain queens (GLM, p > 0.05), but for high strain queens, there was a significant positive relationship between mass and ovariole number (GLM, F1,58 = 9.81, p = 0.0027) with on average an increase of 0.684 ovarioles for every mg increase in mass (std error = 0.218, t = 3.13).
Colony-reared AHB and EHB queens
For mass, there was a significant genotype x rearing environment interaction; EHB queens were smaller on average than AHB queens, but only when reared by EHB workers (GLM, F1,152 = 4.43, p = 0.037; effect = -15.83 mg, s.e. = 7.52, t = -2.105; see the electronic supplementary material, figure S5). For ovariole number in AHB and EHB queens, there were no effects of genotype or rearing environment (GLM, df = 153, all p > 0.05). With all terms in the full model for total ovariole number, mass, genotype x mass, and rearing environment x mass were all significant (GLM, df = 148, p < 0.05), but these effects were not stable and when non-significant terms were sequentially removed no factors remained significant. When we looked at each genotype separately, there were no significant effects for EHB queens (GLM, df = 79, all p > 0.05). In contrast, for AHB queens, mass was positively associated with ovariole number (GLM, F1, 69 = 7.73, p = 0.0070), with an increase of 1.18 ovarioles (s.e. = 0.338, t = 3.49) for every mg increase in mass; and there was a rearing environment x mass interaction (GLM, F1,69 = 4.77, p = 0.032), because there was a relationship between mass and ovariole number when AHB queens were reared in a AHB environment but not in a EHB environment. However this relationship depended largely on a few individuals with small body and ovary size.
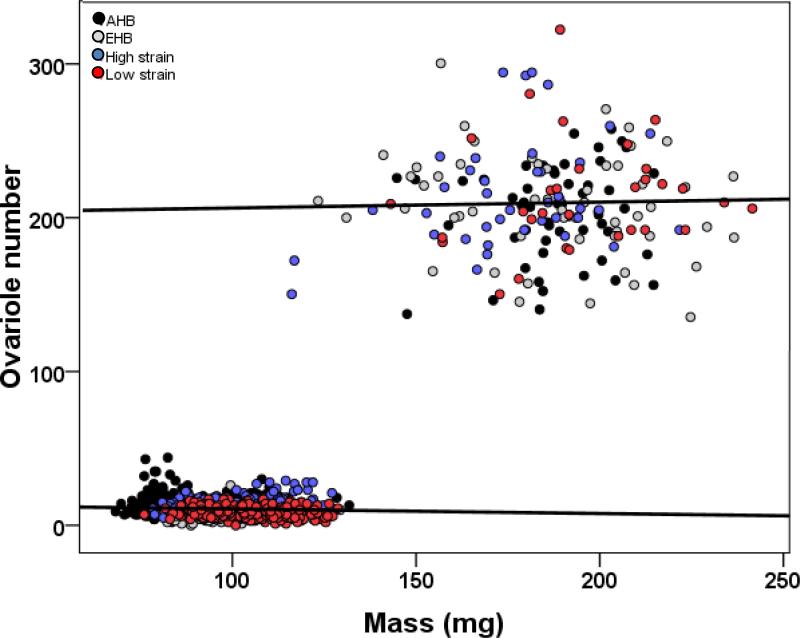
When pooling all genotypes, there was no relationship between body size and ovary size for hive-reared workers (Spearman's rank correlation; ρ = -0.042, p = 0.20, n = 938) but there was a positive body-ovary size relationship in hive-reared queens (spearman's rank correlation; ρ = 0.188, p = 0.002, n = 260) (figure 2).
Fig. 2.
Body size-ovary size relationship across colony-reared workers and queens. AHB (dark gray), EHB (light gray), high (blue) and low (red) pollen hoarding strains for workers (lower left cloud of points) and queens (upper right cloud of points).
Laboratory-reared females
When the influence of social environment was removed by in vitro rearing there were main effects of genotype (GLM, F1,558 = 16.81, p < 0.0001) and food treatment (GLM, F1, 558 = 207.85, p < 0.0001), and a genotype x treatment interaction (GLM, F3, 558 = 17.62, p < 0.0001; F see the electronic supplementary material, figure S6) on mass. This interaction is a genotype x environment interaction (GxE) in which different genotypes display different patterns of phenotypic plasticity in response to the two different nutritional environments we provided. For ovariole number, there were main effects of genotype (GLMM, df=3, χ2 =5246.6, p < 0.0001) and food treatment (GLMM, df=1, χ2 =3735.1, p < 0.0001), and a genotype x treatment interaction (GLMM, df = 3, χ2 = 1035.8, p < 0.0001; see the electronic supplementary material, figure S6). As for mass, this is GxE, indicating the different genotypes respond differently to the nutritional environments for ovary size.
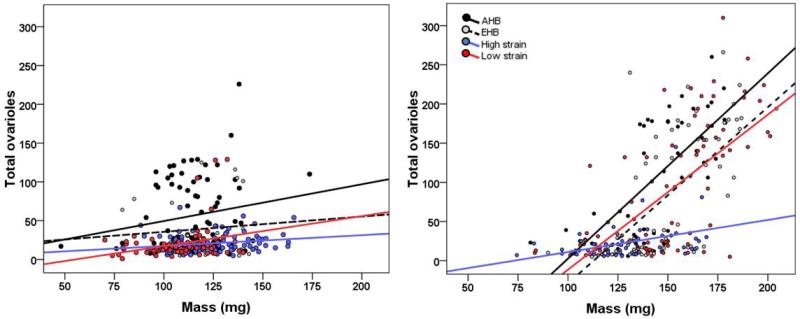
In the full model also including mass, all main and two- and three-way interaction effects involving genotype, treatment, and mass were significant (GLM, all p < 0.01), indicating that the genotypes had different relationships between mass and ovariole number for each treatment (figure 3). Within the worker diet treatment, there was only a significant effect of mass (GLM, F1, 286 = 14.34, p < 0.001), indicating that all genotypes had a similar positive relationship between mass and ovariole number (figure 3). In contrast, within the queen diet treatment, there were significant effects of genotype (GLM, F3, 216 = 4.35, p = 0.005), mass (GLM, F1, 216 = 97.5, p < 0.0001), and genotype x mass interaction (GLM, F3, 216 = 6.19, p < 0.001), indicating that for the queen diet, the genotypes had different relationships between mass and ovariole number (figure 3). Similarly, when we used a regression model with genotype, mass, and their interaction, but without diet treatment, there was an effect of genotype (GLM, F3, 453 = 7.92, p < 0.0001), mass (GLM, F1, 453 = 197.92, p < 0.0001), and genotype x mass interaction (GLM, F3, 453 = 11.72, p < 0.0001). Figure 3 shows that high strain individuals responded differently to the food treatment than the other genotypes; specifically, ovary size did not increase as rapidly with mass for high strain bees fed the queen diet.
Fig. 3.
Body size-ovary size relationship across in vitro reared females. AHB (dark grey), EHB (light grey), high strain (blue), and low strain (red) larvae reared in vitro on a worker (left panel) or queen (right panel) diet.
Over all genotypes and the two food treatments, ovariole number was strongly positively correlated with degree of mandibular notch (a queen character; Spearman's rank correlation, ρ = 0.801, p < 0.001, n = 516), strongly negatively correlated with degree of corbicula development (a worker character; Spearman's rank correlation, ρ = −0.830, p < 0.001, n = 505), and less strongly positively correlated with mass (Spearman's rank correlation, ρ = 0.584, p < 0.001, n = 525). Using scores for ovary size, degree of corbicula development, and degree of mandibular notch development, we defined classes of individuals as worker or queen phenotypes if they fit within the ranges of hive-reared workers and queens, or “intercastes” if they had intermediate values for these three traits. The four genotypes produced different proportions of workers, intercastes, and queens when fed a “queen diet” (Table 1; χ2 = 76.84, df = 6, p < 0.001). Most notably, across the two in vitro treatments, only 6.2% of high strain larvae developed into an intercaste phenotype, while 25% of low strain larvae and on average 44% of EHB and AHB larvae developed into an intercaste or queen phenotype (table 1). Furthermore, within the “worker diet” treatment, there was variation between strains in the proportion of workers, intercastes, and queens produced (χ2 = 53.296, df = 6, p < 0.001); in particular AHB produced a higher proportion of queens and intercastes (0.4, n = 75; see the left panel of figure 3 and note that there are many AHB females between approximately 100-125 mg but with more than 100 ovarioles) relative to EHB (0.174, n = 23), high strain (0.0313, n = 96), and low strain (0.0617, n = 81), indicating that AHB larvae were more likely to initiate the development of queen characters relative to the other strains, even under relatively low nutrition conditions.
Table 1.
Proportion of worker, intercaste, and queen phenotypes produced by AHB, EHB, high, and low pollen hoarding strain larvae reared in vitro on a queen diet.
| Worker | intercaste | Queen | N | |
|---|---|---|---|---|
| AHB | 0.551 | 0.305 | 0.144 | 118 |
| EHB | 0.576 | 0.2 | 0.224 | 85 |
| Low strain | 0.75 | 0.109 | 0.141 | 156 |
| High strain | 0.938 | 0.0621 | 0 | 161 |
High strain larvae were less likely than the other strains to develop into intercaste or queen phenotypes. Queen and worker phenotypes were defined by ovary size, degree of mandibular notch and corbiculae, see Methods.
Discussion
Social evolution in the honey bees (genus Apis) has led to a dramatic elaboration of queen-worker dimorphism so that only two relatively discrete sets of trait-constellations that span a fraction of the possible phenotypic space are naturally expressed. Our results demonstrate that differences between honey bee lineages in developmental plasticity for body size and ovary size and body-ovary size allometry result from differences in the nutritional environment provided by nurse workers and differences in larval developmental responses to nutritional environmental inputs. Our interpretation of these findings is that our pairs of study lineages have diverged for larval and nurse components that control the expression of developmental plasticity and allometry. We suggest that these components were also available for selection to act on during the evolution of queen-worker dimorphism, and that the evolution of queen-worker dimorphism involved the developmental integration and coevolution of these components.
The expression of other polyphenisms besides queen-worker caste dimorphism also depends on social inputs, e.g., dispersal morph in the locust Schistocerca gregaria (Maeno & Tanaka, 2008), head morph in the salamander Hynobius retardatus (Michimae et al., 2009), and tadpole morph in the spadefoot toad Spea spp. (Pfennig & Frankino, 1997, Martin & Pfennig, 2010). In some cases both social inputs and developmental responses have been shown to be heritable and can respond to selection, e.g., male horn length in the horn-dimorphic dung beetle Onthophagus taurus (Moczek, 1998, Moczek & Nijhout, 2002, Hunt & Simmons, 2002). Thus, developmental evolution may often involve the coevolution and integration of social inputs and plastic developmental responses.
Confirming our previous results (Linksvayer et al., 2009a), worker ovary size differences between the high and low pollen hoarding strains was determined by an interaction between the genotype of focal individuals and the genotypic makeup of the rearing environment. Similarly, body size differences between AHB and EHB workers and queens were determined by an interaction between genotype and social environment. These results emphasize that the expression of social insect phenotypes depend on the combination of focal individual genotype and the genotypic composition, or “sociogenome” of the colony (Linksvayer, 2007, Linksvayer et al., 2009a).
The relationship between body size and ovary size expressed by high strain workers, but not low strain workers, also depended on the rearing environment (figure 1). It seems that high strain larvae are more sensitive than low strain larvae, in terms of the resulting adult ovary size over the range of nutritional environments provided by low strain nurses. Besides food quantity, high and low strain nurses may provide qualitatively different food, or may differ in the timing of food delivery. These different parameters may depend on the specific signaling and response interactions that occur between different nurse-larvae genotypic combinations (Kolliker et al., 2005, Linksvayer et al., 2009a), resulting in changes in developmental plasticity and expressed allometry. Regardless the mechanism, the differences we observed in worker allometry between the high and low strains indicate that the strains have diverged for expressed body-ovary allometry, likely as a result of the artificial, colony-level selection program (Linksvayer et al., 2009a). While high and low strain workers differed in their sensitivity to the rearing environment for their expressed body-ovary size relationship, AHB and EHB workers showed consistent changes in the body-ovary relationship depending on the rearing environment (electronic supplementary material, figure S3). We also found evidence that the rearing environment may play a role in the different body-ovary size relationships expressed by the different queen genotypes.
Our in vitro feeding results showed that larvae of the different genotypes also respond differently to the same nutritional environment, when there was no potential for social control of development. While low strain, EHB, and AHB larvae responded to the high food, “queen diet” treatment with increased body mass and increased ovary size, high strain larvae grew larger but did not respond with a similar increase in ovary size (figure 3). Indeed the low strain, EHB, and AHB produced many queen and queen-like phenotypes, whereas none of the high strain larvae developed queen-like phenotypes (Table 1). These results show that the artificial colony-level selection program has also shaped components of the developmental response of larvae to their nutritional environment that affect the body size-ovary size relationship spanning the full range of possible female body sizes. The genotypes also differed in their likelihood to develop queen-like phenotypes when fed the “worker diet”, and in particular many AHB individuals developed queen-like traits. In a previous study we found that some genotypes of colony-reared AHB workers had large queen-like ovaries, and furthermore the expression of this large ovary phenotype was conditional on the social rearing environment (Linksvayer et al., 2009b). Thus, segregating variation for caste-related traits, and body-ovary allometry, may be relatively common in honey bee populations. Indeed, recent theory indicates that relatively high levels of genetic variation for caste can be maintained, even when variants are deleterious, by mutation-kin selection balance (Van Dyken et al., 2011).
Within three of the four lineages, we found that body size and ovary size were not consistently correlated within queens and workers, suggesting that it is possible for these traits to be dissociated and independently canalized. The exception was the two sources of high pollen hoarding strain bees, which displayed different patterns of plasticity and allometry. The pollen hoarding strain bees used were derived from generation 33 of artificial selection on commercial EHB stocks, demonstrating that plasticity and allometry can change rapidly. Similarly, studies with Onthophagus beetles using artificial selection (Emlen, 1996) and comparisons between natural and recently introduced populations show that allometries can evolve rapidly (Moczek & Nijhout, 2003). However, the rapid change in plasticity for high pollen hoarding strain worker traits may explain the disrupted queen development we observed in the high strain, suggesting that the developmental mechanisms linking the expression of queen and worker traits may constrain their independent evolution, at least over short evolutionary time scales (Tomkins & Moczek, 2009). Over longer evolutionary time scales it may be possible for the relationship between worker body size and ovary size to evolve without disrupting queen development. Indeed, EHB and AHB lineages initially diverged approximately 1 million years ago (Whitfield et al., 2006), and while EHB and AHB lineages show similar differences as the high and low pollen hoarding strains in terms of worker body size and ovary size (i.e. both AHB and high pollen hoarding strains have smaller worker mass but larger worker ovaries), the EHB and AHB lineages both produced normal queen phenotypes when reared in vitro (Table 1).
It appears that high strain females do not initiate queen development as readily as low strain, EHB, and AHB females, suggesting that high strain larvae are less sensitive to nutritional cues that promote queen development. Why are high strain larvae seemingly more sensitive to the rearing environment over the range of worker phenotypic space but less sensitive to the rearing environment over the broader range extending to queen phenotypic space?
We propose that our data can point to underlying developmental mechanisms that also allow us to explain the different sensitivities that high strain larvae show across the worker and queen phenotypic spaces. Recent studies suggest that developmental growth is regulated in the holometabolous insects by specific sensing mechanisms for body size. Central to this system is insulin/insulin-like signaling (IIS), a nutrient sensitive signaling cascade that, together with the TOR (target of rapamycin) complex, affects the synthesis and release of ecdysteroid molting hormones and influence patterns of tissue- and organismal growth in many organisms, perhaps including the honey bee (Walkiewicz & Stern, 2009, Patel et al., 2007). Organ size and body size are generally related, but high strain workers retain a larger ovary inside a smaller body. High strain bees also differ from low stain bees in the expression of several IIS and TOR associated genes (Wang et al., 2009), and during early development they show a different pattern of ecdysteroid release than low strain workers (Amdam et al., 2010). These findings suggest that the body-ovary allometry that sets high strain bees apart from low stain bees and EHB could emerge from a different sensing of body size during development.
Queen larvae grow faster and attain a larger final size than worker-destined individuals, so that successful sensing of a critical size during development is likely central to the expression of queen traits (Patel et al., 2007). Although large size is attained by high strain bees reared in vitro, these bees fail to express queen traits. This result is consistent with the suggestion that body size may be differently interpreted by the high selected strain during development.
Thus, we propose that retention of a larger ovary in high strain worker bees can be achieved by disrupting the body-ovary size relationship such that a larger ovary is achieved in a smaller body. The disruption of the body-ovary relationship via changes in the sensitivity of body size and ovary size to the nutritional environment seems to have been coupled with nurse-worker regulation of the rearing environment such that a limited range of worker phenotypes are produced under natural conditions. This disruption, however, also affects the queen phenotype in high strain bees, in that it becomes more difficult to express. We propose that this phenotypic outcome has been maintained because the artificial selection program acts more strongly on worker traits than queen traits. Only a handful of queens need to ever be produced and be functional to continue the artificial selection lines, resulting in relatively relaxed selection on queen traits. In contrast, worker traits mainly determine the amount of pollen collected and stored in the hive so that artificial selection on pollen hoarding likely acts more strongly on worker traits. Relatively relaxed selection on queen traits may also occur naturally in swarm-founding species, where queens do not initiate colonies alone.
We reared larvae in vitro on two discrete quantities of food, but we ended up with adult females that were more variable than colony-reared queens and workers, indicating that nurse bees are better at regulating expressed queen-worker dimorphism than we are (compare Fig. 2 and Fig. 3). By feeding larvae continuously (instead of a single time in our in vitro protocol), nurse workers can tightly regulate available food over the course of development. Nurse workers can also regulate development by removing individuals that express intermediate phenotypes, so that only discrete queen and worker phenotypes are produced (Weaver, 1957, Woyke, 1971, Dedej et al., 1998, Hatch et al., 1999).
For many species that express a polyphenism, there is a step-like relationship between body size and the polyphenic trait, so that only discrete alternate morphs are produced across the full range of body sizes (Nijhout, 2003). In contrast, our in vitro results suggest that discrete queen and worker morphs result mainly from discrete, nurse-controlled nutritional environments, not because of an underlying step-like relationship between body size and ovary size that converts variable larval nutrition into discrete alternate phenotypes. As shown in figure 3, a continuous range of ovary size and body size phenotypes was produced in the absence of social control, with the in vitro queen diet. Thus, under natural colony conditions, only the extreme ends of the full phenotypic space are normally expressed, and the relationship between body size and ovary size that we observed in vitro is typically suppressed, so that ovary size is relatively canalized over the range of worker and queen body sizes produced (see Fig. 2). The apparent lack of a steep step-like relationship highlights the importance of social control for ensuring the production of discrete queen and worker morphs and may also indicate that social control played an important role during the early evolution as well as elaboration of honey bee queen-worker dimorphism. That is, the evolution of a steep, step-like relationship between body size and ovary size may have been unnecessary precisely because there already was relatively strict social control. Further study of the relative importance of social control of larval development and larval developmental responses to social inputs in other social insect lineages will help to clarify the various roles that these components have played during the evolution of eusociality.
Supplementary Material
Acknowledgements
TAL was supported by a National Science Foundation (NSF) postdoctoral fellowship. REP was supported by the National Institute of Aging (NIA P01 AG22500). TAL, REP, and GVA would also like to thank the Wissenschaftskolleg zu Berlin for support while writing. Thanks to the Harrison lab for use of the microtome, MK Fondrk for advice and beekeeping help, and thanks to two anonymous reviewers for their helpful comments.
References
- Allsopp MH, Calis JNM, Boot WJ. Differential feeding of worker larvae affects caste characters in the Cape honeybee, Apis mellifera capensis. Behavioral Ecology and Sociobiology. 2003;54:555–561. [Google Scholar]
- Amdam GV, Csondes A, Fondrk MK, Page RE. Complex social behaviour derived from maternal reproductive traits. Nature. 2006;439:76–78. doi: 10.1038/nature04340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amdam GV, Page RE, Fondrk K, Brent CS. Hormone response to bidirectional selection on social behavior. Evolution and Development. 2010;12:425–433. doi: 10.1111/j.1525-142X.2010.00429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KE, Linksvayer TA, Smith CR. The causes and consequences of genetic caste determination in ants (Hymenoptera: Formicidae). Myrmecological News. 2008;11:119–132. [Google Scholar]
- Barchuk AR, Cristino AS, Kucharski R, Costa LF, Simoes ZLP, Maleszka R. Molecular determinants of caste differentiation in the highly eusocial honeybee Apis mellifera. Bmc Developmental Biology. 2007;7 doi: 10.1186/1471-213X-7-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beekman M, Calis JNM, Boot WJ. Insect behaviour - Parasitic honeybees get royal treatment. Nature. 2000;404:723–723. doi: 10.1038/35008148. [DOI] [PubMed] [Google Scholar]
- de Azevedo SV, Hartfelder K. The insulin signaling pathway in honey bee (Apis mellifera) caste development - differential expression of insulin-like peptides and insulin receptors in queen and worker larvae. Journal of Insect Physiology. 2008;54:1064–1071. doi: 10.1016/j.jinsphys.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Dedej S, Hartfelder K, Aumeier P, Rosenkranz P, Engels W. Caste determination is a sequential process: effect of larval age at grafting on ovariole number, hind leg size and cephalic volatiles in the honey bee (Apis mellifera carnica). Journal of Apicultural Research. 1998;37:183–190. [Google Scholar]
- Emlen DJ. Artificial selection on horn length body size allometry in the horned beetle Onthophagus acuminatus (Coleoptera: Scarabaeidae). Evolution. 1996;50:1219–1230. doi: 10.1111/j.1558-5646.1996.tb02362.x. [DOI] [PubMed] [Google Scholar]
- Emlen DJ, Lavine LC, Ewen-Campen B. On the origin and evolutionary diversification of beetle horns. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8661–8668. doi: 10.1073/pnas.0701209104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JD, Wheeler DE. Differential gene expression between developing queens and workers in the honey bee, Apis mellifera. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:5575–5580. doi: 10.1073/pnas.96.10.5575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch S, Tarpy DR, Fletcher DJC. Worker regulation of emergency queen rearing in honey bee colonies and the resultant variation in queen quality. Insectes Sociaux. 1999;46:372–377. [Google Scholar]
- Hölldobler B, Wilson EO. The Ants. Harvard University Press; Cambridge, MA: 1990. [Google Scholar]
- Hunt J, Simmons LW. The genetics of maternal care: Direct and indirect genetic effects on phenotype in the dung beetle Onthophagus taurus. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:6828–6832. doi: 10.1073/pnas.092676199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarau S, van Veen JW, Twele R, Reichle C, Gonzales EH, Aguilar I, Francke W, Ayasse M. Workers Make the Queens in Melipona Bees: Identification of Geraniol as a Caste Determining Compound from Labial Glands of Nurse Bees. Journal of Chemical Ecology. 2010;36:565–569. doi: 10.1007/s10886-010-9793-3. [DOI] [PubMed] [Google Scholar]
- Kaftanoglu O, Linksvayer TA, Page RE. Rearing honey bees (Apis mellifera L.) in vitro: effects of feeding intervals on survival and development. Journal of Apicultural Research. 2010;49:311–317. [Google Scholar]
- Kolliker M, Brodie ED, Moore AJ. The coadaptation of parental supply and offspring demand. American Naturalist. 2005;166:506–516. doi: 10.1086/491687. [DOI] [PubMed] [Google Scholar]
- Laidlaw HH, Page RE. Queen Rearing and Bee Breeding. Wicwas Press; Cheshire, Connecticut: 1997. [Google Scholar]
- Linksvayer TA. Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evolution. 2006;60:2552–2561. doi: 10.1554/06-011.1. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA. Ant species differences determined by epistasis between brood and worker genomes. PLoS ONE. 2007;2:e994, 1–5. doi: 10.1371/journal.pone.0000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linksvayer TA, Fondrk MK, Page RE. Honeybee Social Regulatory Networks Are Shaped by Colony-Level Selection. American Naturalist. 2009a;173:E99–E107. doi: 10.1086/596527. [DOI] [PubMed] [Google Scholar]
- Linksvayer TA, Rueppell O, Siegel A, Kaftanoglu O, Page RE, Amdam GV. The Genetic Basis of Transgressive Ovary Size in Honeybee Workers. Genetics. 2009b;183:693–707. doi: 10.1534/genetics.109.105452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linksvayer TA, Wade MJ. The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Quarterly Review of Biology. 2005;80:317–336. doi: 10.1086/432266. [DOI] [PubMed] [Google Scholar]
- Maeno K, Tanaka S. Maternal effects on progeny size, number and body color in the desert locust, Schistocerca gregaria: Density- and reproductive cycle-dependent variation. Journal of Insect Physiology. 2008;54:1072–1080. doi: 10.1016/j.jinsphys.2008.04.010. [DOI] [PubMed] [Google Scholar]
- Martin RA, Pfennig DW. Maternal Investment Influences Expression of Resource Polymorphism in Amphibians: Implications for the Evolution of Novel Resource-Use Phenotypes. Plos One. 2010;5 doi: 10.1371/journal.pone.0009117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michener CD. The Social Behavior of Bees. Harvard University press; Cambridge: 1974. [Google Scholar]
- Michener CD. The Bees of the World. The Johns Hopkins University Press; Baltimore (MD): 2000. [Google Scholar]
- Michimae H, Nishimura K, Tamori Y, Wakahara M. Maternal effects on phenotypic plasticity in larvae of the salamander Hynobius retardatus. Oecologia. 2009;160:601–608. doi: 10.1007/s00442-009-1319-8. [DOI] [PubMed] [Google Scholar]
- Moczek AP. Horn polyphenism in the beetle Onthophagus taurus: larval diet quality and plasticity in parental investment determine adult body size and male horn morphology. Behavioral Ecology. 1998;9:636–641. [Google Scholar]
- Moczek AP, Nijhout HF. Developmental mechanisms of threshold evolution in a polyphenic beetle. Evolution & Development. 2002;4:252–264. doi: 10.1046/j.1525-142x.2002.02014.x. [DOI] [PubMed] [Google Scholar]
- Moczek AP, Nijhout HF. Rapid evolution of a polyphenic threshold. Evolution & Development. 2003;5:259–268. doi: 10.1046/j.1525-142x.2003.03033.x. [DOI] [PubMed] [Google Scholar]
- Nijhout HF. Development and evolution of adaptive polyphenisms. Evolution & Development. 2003;5:9–18. doi: 10.1046/j.1525-142x.2003.03003.x. [DOI] [PubMed] [Google Scholar]
- Osborne KE, Oldroyd BP. Possible causes of reproductive dominance during emergency queen rearing by honeybees. Animal Behaviour. 1999;58:267–272. doi: 10.1006/anbe.1999.1139. [DOI] [PubMed] [Google Scholar]
- Pankiw T, Tarpy DR, Page RE. Genotype and rearing environment affect honeybee perception and foraging behaviour. Animal Behaviour. 2002;64:663–672. [Google Scholar]
- Patel A, Fondrk MK, Kaftanoglu O, Emore C, Hunt G, Frederick K, Amdam GV. The Making of a Queen: TOR Pathway Is a Key Player in Diphenic Caste Development. Plos One. 2007;2:e509. doi: 10.1371/journal.pone.0000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig DW, Frankino WA. KIN-mediated morphogenesis in facultatively cannibalistic tadpoles. Evolution. 1997;51:1993–1999. doi: 10.1111/j.1558-5646.1997.tb05120.x. [DOI] [PubMed] [Google Scholar]
- Presnell JK, Martin P. Humanson's Animal Tissue Techniques. fifth edition Johns Hopkins University Press; Baltimore: 1997. [Google Scholar]
- Rinderer TE, Sylvester HA, Collins AM, Pesante D. IDENTIFICATION OF AFRICANIZED AND EUROPEAN HONEY BEES APIS-MELLIFERA EFFECTS OF NURSE-BEE GENOTYPE AND COMB SIZE. Bulletin of the Entomological Society of America. 1986;32:150–152. [Google Scholar]
- Schwander T, Lo N, Beekman M, Oldroyd BP, Keller L. Nature versus nurture in social insect caste differentiation. Trends in Ecology & Evolution. 25:275–282. doi: 10.1016/j.tree.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Tomkins JL, Moczek AP. PATTERNS OF THRESHOLD EVOLUTION IN POLYPHENIC INSECTS UNDER DIFFERENT DEVELOPMENTAL MODELS. Evolution. 2009;63:459–468. doi: 10.1111/j.1558-5646.2008.00563.x. [DOI] [PubMed] [Google Scholar]
- Van Dyken JD, Linksvayer TA, Wade MJ. Kin selection-mutation balance: a model for the origin, maintenance, and consequences of social cheating. American Naturalist. 2011;177:288–300. doi: 10.1086/658365. [DOI] [PubMed] [Google Scholar]
- Walkiewicz MA, Stern M. Increased insulin / insulin growth factor signaling advances the onset of metamorphosis in Drosophila. PLoS ONE. 2009;4:e5072. doi: 10.1371/journal.pone.0005072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Amdam GV, Rueppell O, Wallrichs MA, Fondrk MK, Kaftanoglu O, Page RE. PDK1 and HR46 Gene Homologs Tie Social Behavior to Ovary Signals. Plos One. 2009;4 doi: 10.1371/journal.pone.0004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver N. Rearing of Honeybee Larvae on Royal Jelly in the Laboratory. Science. 1955;121:509–510. doi: 10.1126/science.121.3145.509. [DOI] [PubMed] [Google Scholar]
- Weaver N. Experiments on dimorphism in the female honey bee. Journal of Economic Entomology. 1957;50:759–761. [Google Scholar]
- Wheeler DE. Developmental and physiological determinants of caste in social Hymenoptera: evolutionary implications. American Naturalist. 1986;128:13–34. [Google Scholar]
- Wheeler DE, Buck N, Evans JD. Expression of insulin pathway genes during the period of caste determination in the honey bee, Apis mellifera. Insect Molecular Biology. 2006;15:597–602. doi: 10.1111/j.1365-2583.2006.00681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield CW, Behura SK, Berlocher SH, Clark AG, Johnston JS, Sheppard WS, Smith DR, Suarez AV, Weaver D, Tsutsui ND. Thrice out of Africa: Ancient and recent expansions of the honey bee, Apis mellifera. Science. 2006;314:642–645. doi: 10.1126/science.1132772. [DOI] [PubMed] [Google Scholar]
- Winston ML. The Biology of the Honey Bee. Harvard University Press; Cambridge, M.A.: 1987. [Google Scholar]
- Wolf JB, Brodie ED., III The coadaptation of parental and offspring characters. Evolution. 1998;52:299–308. doi: 10.1111/j.1558-5646.1998.tb01632.x. [DOI] [PubMed] [Google Scholar]
- Woyke J. Correlations between the age at which honeybee brood was gafted, characteristics of the resultant queens, and results of insemination. Journal of Apicultural Research. 1971;10:45–55. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.