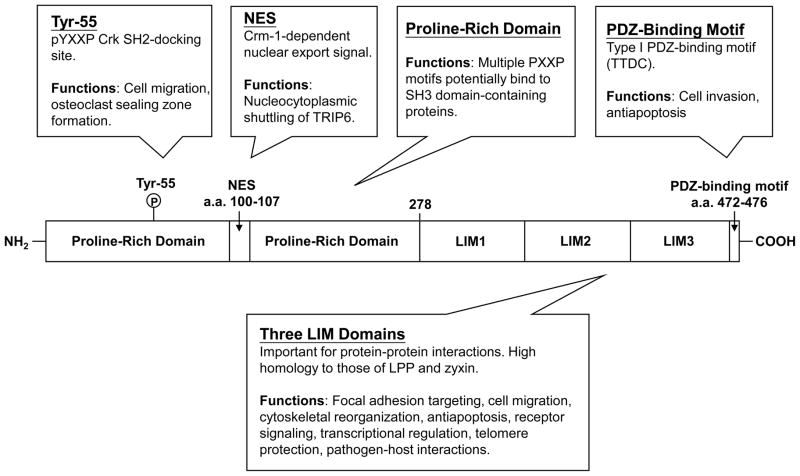
Figure 1. Schematic representation of TRIP6.
The N-terminal half of TRIP6 contains a proline-rich region with multiple PXXP motifs, a Crm-1-dependent nuclear export signal, and a phosphotyrosine-55-directed pYXXP motif that serves as a docking site for Crk SH2 domain. The carboxyl-terminal half of TRIP6 contains three tandem LIM domains and a PDZ-binding motif. These multiple elements allow TRIP6 to serve as an adaptor for a wide variety of partners with diverse functional outcomes.