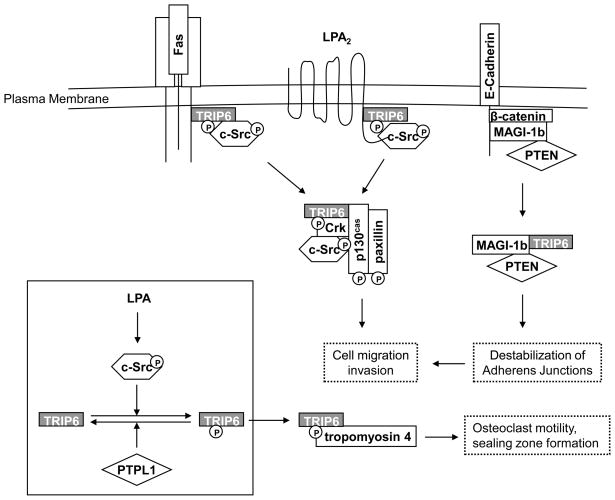
Figure 2. TRIP6 promotes cell motility, invasion and osteoclast sealing zone formation through c-Src-mediated phosphorylation and the interaction with a number of cell-surface receptors and signaling molecules involved in these dynamic processes.
TRIP6 binds to the juxtamembrane domains of the Fas/CD95 and LPA2 receptors upon stimulation with FasL and LPA, respectively. This induces phosphorylation of TRIP6 at Tyr-55 by c-Src, an event reversed by PTPL1, and triggers its association with Crk and various regulators of actin remodeling, leading to an increase in cell motility and promoting osteoclast sealing zone formation. TRIP6 also competes with β-catenin for binding to the MAGI-1b/PTEN signalsome. As a result, this destabilizes adherens junctions and increases cell invasiveness.