Abstract
This investigation sought to determine if aging affected adaptations of the neuromuscular junction (NMJ) to exercise training. Twenty young adult (8 mo), and 20 aged (24 mo) rats were assigned to either a program of treadmill exercise, or sedentary conditions. Following the 10 week experimental period, rats were euthanized, and soleus and plantaris muscles were removed and frozen. Longitudinal sections of the muscles were fluorescently stained to visualize pre-synaptic nerve terminals, and post-synaptic endplates on both slow- and fast-twitch fibers. Images were collected with confocal microscopy and quantified. Muscle cross-sections were histochemically stained to assess muscle fiber profiles (size and fiber type). Our analysis of NMJs revealed a high degree of specificity and sensitivity to aging, exercise training, and their interaction. In the soleus, slow-twitch NMJs demonstrated significant (P ≤ 0.05) training-induced adaptations in young adult, but not aged rats. In the fast-twitch NMJs of the soleus, aging, but not training was associated with remodeling. In the plantaris, aging but not training, remodeled the predominant fast-twitch NMJs, but only pre-synaptically. In contrast, the slow-twitch NMJs of the plantaris displayed morphologic adaptations to both aging and exercise in pre- and post-synaptic components. Muscle fiber profiles indicated that changes in NMJ size were unrelated to adaptations of their fibers. Our data show that aging interferes with the ability of NMJs to adapt to exercise training. Results also reveal complexity in the coordination of synaptic responses among different muscles, and different fiber types within muscles, in their adaptation to aging and exercise training.
Keywords: synapse, endplate, nerve terminal, neuromuscular junction, muscle fiber, exercise
Neuromuscular fatigue has long been identified as a limiting factor in the performance of exercise training. In particular, the neuromuscular transmission of electrical impulses across the neuromuscular junction (NMJ) plays a role in fatigue (Belluardo et al., 2001; Panenic et al., 1999; Sieck and Prakash, 1995). In young adults, participating in a program of endurance training elicits adaptations of the NMJ including increased pre- and post-synaptic size, and ability to release and bind neurotransmitter that enable this vital synapse to improve its ability to stave off neuromuscular transmission failure (Deschenes et al., 1993; Andonian and Fahim, 1987; Dorlochter et al., 1991). This, in turn, allows exercise sessions to continue for longer durations at a sustained intensity. And although it is known that aging is associated with functional and morphological adaptations of the NMJ (Andonian and Fahim, 1987; Prakash and Sieck, 1998), it is unclear at this point whether those alterations impair the potential of the NMJ to experience the same degree of training induced remodeling observed in young adults. Accordingly, the aim of the present investigation was to determine whether aging alters the capacity of the neuromuscular system – both NMJs and the muscle fibers they innervate – to undergo adaptations to exercise training. It was hypothesized that aging would, in fact, modify NMJ adaptations derived from exercise training, as well as the remodeling experienced by the muscle fibers upon which NMJs reside.
Experimental procedures
Subjects
Twenty young adult (8 months old) and 20 aged (24 months old) male Fischer 344 rats were purchased from the National Institute on Aging (NIA) Colonies. The average lifespan of male Fischer 344 rats when housed and cared for at the NIA colonies is 25.5 months (Turturro et al., 1999). Thus, at 24 months of age, the rats used here began the investigation having lived 94% of their life expectancy. Relative to the current life expectancy of men in the United States of 75.2 years (Arias, 2006), the aged rats of this project could be considered the equivalent of 71 year old men. By the same calculations, young adult rats (8 months of age) would be the equivalent of 24 year old men.
Rats from both age categories were assigned to either exercise trained, or control groups. Both young trained (N=10) and aged trained (N=10) rats participated in the same 10 week treadmill running program. The program began with training sessions of 15 min at a speed of 7.5 meters/min at a 0% grade completed 5 days/week. Treadmill speed and duration of exercise sessions were gradually increased such that by the final week, young and aged rats ran for 60 min per session at a speed of 15 meters/min while maintaining a 0% grade. Increments in exercise speed and duration were determined by the tolerance of aged animals, and replicated by young ones, to ensure that both age groups completed the same training regimen. Exercise tolerance was subjectively determined by visual indications of physical fatigue such as decisively labored strides, heavy panting, and inability to maintain pace. Aged (N=10) and young (N=10) controls were untrained, remaining in their cages throughout the 10 week intervention period. During the course of the intervention period, however, one aged trained rat, and one aged control rat died of apparently natural causes, reducing sample size to 9 for each of those two groups. All animals, regardless of age or treatment group were permitted to eat rat chow and drink water ad libitum, and were housed in a 21–22° C environment on a 12 hour light-dark cycle. All procedures employed had been previously approved by the institutional animal care and use committee in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Tissue preparation and storage
At the conclusion of the 10 week intervention period, rats from all treatment groups were weighed and then euathanized. Immediately following death, hindlimb muscles were dissected out, cleared of fat and connective tissue and frozen at approximate resting length in isopentane chilled with liquid nitrogen. Muscle were then stored at −85° C until analysis. For the present study the soleus and plantaris muscles were selected for study. The soleus is primarily a slow-twitch muscle comprised mainly (~85%) of type I muscle fibers (Delp and Duan, 1996) and serves as the main postural muscle, but is also recruited during running (Dudley et al., 1982; Roy et al., 1985). In contrast, the plantaris is principally (>90%) comprised of type II (fast-twitch) muscle fibers (Delp and Duan, 1996), has little role in posture, but is recruited during locomotor activity (Laughlin and Armstrong, 1982).
Cytofluorescent staining
To visualize NMJs, 50 µm thick longitudinal sections of the middle one-third of the muscle were obtained at −20°C on a cryostat (Cryocut 1800; Reichert-Jung, NuBloch, Germany). To prevent contraction of sections, microscope slides were pretreated in a 3% Ethylenediaminetetraacetic acid solution as previously described (Pearson and Sabarra, 1974). Sections were washed 4 × 15 min in phosphate buffered saline (PBS) containing 1% bovine serum albumin (BSA). Sections were then incubated in a humidified chamber overnight at 4°C in supernatant of the primary antibody RT97 (Developmental Studies Hybridoma Bank, University of Iowa), diluted 1:20 in PBS with 1% BSA. The RT97 antibody reacts with non-myelinated segments of pre-synaptic nerve terminals (Anderton et al., 1982). The next day, sections were washed 4 × 15 in PBS with 1% BSA before incubating for 2 h at room temperature in fluorescein isothiocyanate (FITC) conjugated secondary immunoglobulin (Sigma Chemical, St. Louis, MO) that was diluted 1:150 in PBS with 1% BSA. Sections were then washed 4 × 15 min in PBS with 1% BSA. Following this, sections were incubated in a humidified chamber overnight at 4°C in a solution containing rhodamine conjugated α-bungarotoxin (BTX; Molecular Probes, Eugene, OR) diluted 1:600 in PBS along with either anti-fast (soleus) or anti-slow (plantaris) myosin heavy chain antibody raised in mouse (Sigma Chemical, St. Louis, MO) diluted 1:50. BTX recognizes post-synaptic acetylcholine (ACh) receptors, while the anti-slow, or anti-fast immunogen enabled us to determine whether the endplate resided on a slow- or fast-twitch myofiber. The next day, sections were washed 4 × 15 min in PBS with 1% BSA before incubating them for 1 hr at room temperature in AlexaFluor 647 (Molecular Probes, Eugene, OR) labeled secondary antibody to bind with the anti-slow, or anti-fast primary antibody. Sections were given a final wash (4 × 15 min) before being lightly coated with Pro Long (Molecular probes, Eugene, OR) and having cover slips applied. Slides were then coded with respect to treatment group to allow for blinded evaluation of NMJ morphology and then stored at −20°C until analysis. Examples of cytofluorescent staining of pre- and post-synaptic components of the NMJ are displayed in Figures 1 and 2, respectively. Pre-synaptic variables of NMJs assessed included: 1) number of branches identified at the nerve terminal, 2) the total length of those branches, 3) average length per branch, and 4) branching complexity which, as described by Tomas et al., (Tomas et al., 1990) is derived by multiplying the number of branches by the total length of those branches and dividing that figure by 100. Post-synaptic variables of interest included: 1) total perimeter, or the length encompassing the entire endplate comprised of stained receptor clusters and non-stained regions interspersed within those clusters, 2) stained perimeter, or the composite length of tracings around individual receptor clusters, 3) total area, which includes stained receptors along with non-stained regions interspersed among receptor clusters, 4) stained area, or the cumulative areas occupied by ACh receptor clusters, and 5) dispersion of endplates, which was assessed by dividing the endplate’s stained area by its total area and multiplying by 100. See Figure 3 to observe post-synaptic tracings around ACh receptors that were generated both manually and by software to assess total and stained perimeter lengths, respectively, and areas contained within those perimeter lengths. In this study, pre- to post-synaptic coupling was quantified by dividing the NMJ’s post-synaptic stained area by its total length of nerve terminal branching.
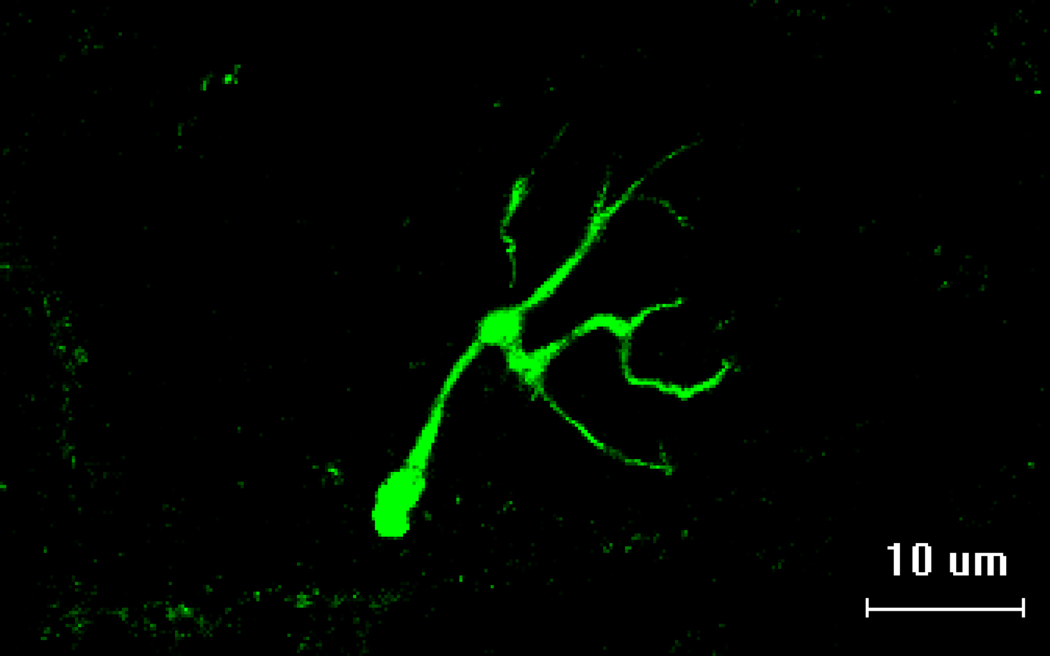
Figure 1.
Representative image of fluorescently stained pre-synaptic nerve terminal branching.
Scale bar equals 10µm.
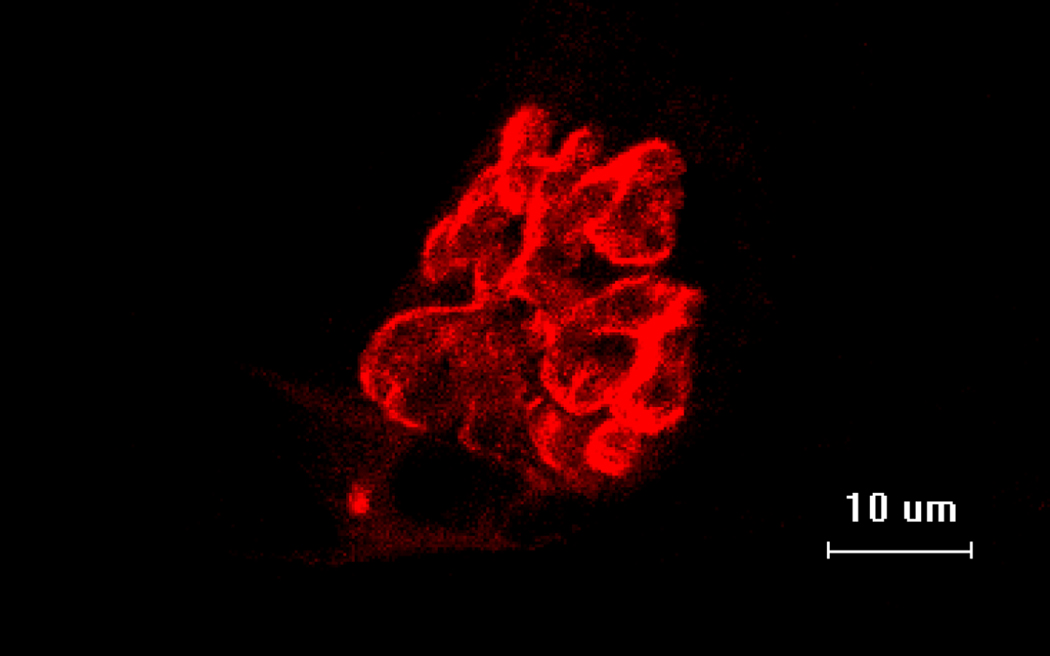
Figure 2.
Representative image of fluorescently stained post-synaptic acetylcholine receptors.
Scale bar equals 10µm.
Figure 3.
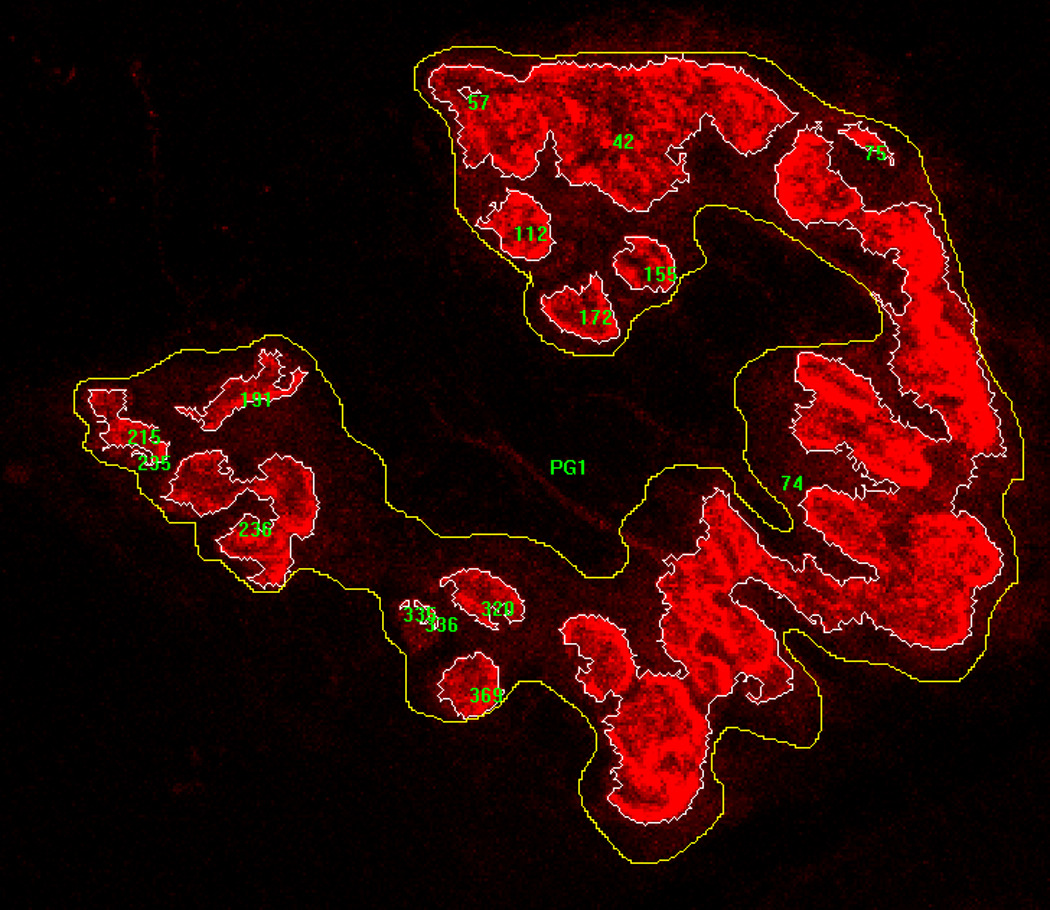
Image of fluorescently stained post-synaptic acetylcholine receptors with examples of manually drawn line surrounding total endplate region (stained areas and unstained areas interspersed within), and computer generated tracings surrounding only stained clusters of receptors. Lines were used to determine perimeter lengths, as well as areas contained within.
Histochemical staining
To quantify muscle fiber profiles, 10 µm thick transverse sections were obtained from the midbelly of the muscle using a cryostat set at −20°C. Sections were stained for myofibrillar ATPase activity following pre-incubation at a pH of either 4.55 (soleus) or 4.40 (plantaris) according to Nemeth and Pette (Nemeth and Pette, 1981). It should be noted that this staining technique allows identification of the three fiber types found in the soleus (types I, IIA and IIX). However, the fourth fiber type included in the plantaris (type IIB) cannot be distinguished from type IIX fibers. Slides were coded so that measurements could be conducted in a blinded fashion regarding treatment group.
Microscopy
An Olympus Fluoview FV 300 confocal system featuring three lasers and an Olympus BX60 fluorescent microscope (Olympus America, Melville, NY) was used to collect images of NMJs and to identify whether they were located on slow or fast-twitch muscle fibers. Using a 100X oil immersion objective, it was initially established that the entire NMJ was within the longitudinal borders of the muscle fiber and that the area of interest was not damaged during sectioning. A detailed image of the entire NMJ was constructed from a z-series of scans taken at 1 µm thick increments. To ascertain the muscle fiber type on which the NMJ resided, a single scan was collected of the fiber using the appropriate wavelength to detect AlexaFluor 647. Digitized, two-dimensional images of NMJs were stored on the system’s hard drive and later quantified with the Image Pro-Plus software (Media Cybernetics, Silver Spring, MD). For the predominant fiber type within the muscle (i.e., slow-twitch in soleus, fast-twitch in plantaris), 10–12 NMJs were imaged and measurements were averaged to represent that muscle. However, in the minority fiber type of the muscle (i.e., fast-twitch in soleus, slow-twitch in plantaris), we were often unable to find the full complement of 10–12 acceptable NMJs to image and a smaller number, but at least 4, were used to average and represent NMJ structure in that muscle.
An Olympus BX41 phase contrast microscope was used to assess myofiber profiles with a 40X objective. Myofiber cross-sectional areas were quantified with the Image Pro-Express software. A random sample of 125–150 myofibers from each muscle was analyzed to determine average myofiber size (i.e., cross-sectional area) and fiber type composition (% of total number of fibers examined) for that muscle.
Statistical analysis
Data are reported as means ± standard error (SE). For body mass, a 2 way analysis of variance with repeated measures was used, along with a Tukey post-hoc analysis when indicated, to identify between group differences and the effects of the 10 week intervention period. All other variables of interest were assessed with 2 way analysis of variance with main effects of age (young, and aged), and treatment (control, and trained). In the event of a significant F-ratio, a Tukey post-hoc test was performed to identify pair-wise differences. In all cases, statistical significance was established at P ≤ 0.05.
Results
Body mass
Our data regarding body mass indicate that there was no difference between young and aged controls at the start of the 10 week intervention period, and in neither age group was there a significant difference between rats assigned to control and trained groups. This enabled us to determine the effects of aging, as well as training, without pre-existing differences in body mass. But in examining pre- to post-intervention data, it was discovered that body mass significantly changed in all four experimental groups over the 10 week program. However, while the aged trained rats experienced a significant decline in body mass, the other three groups significantly gained body mass over that time. And at the post-intervention time point – when muscle samples were collected – young controls were significantly heavier than all other groups, although there was no difference between young and aged trained animals. Thus, any differences in NMJ or muscle fiber morphology between young and aged trained rats cannot be attributed to differences in whole animal size. Data regarding body mass are presented in Table 1.
Table 1.
Body mass (grams) of young adult and aged rats before and after 10 week intervention.
| Group | Pre-intervention | Post-intervention |
|---|---|---|
| Young control (N=10) | 379.6 ± 7.1 | 466.8 ± 10.9‡ |
| Young trained (N=10) | 369.1 ± 6.7 | 379.3 ± 8.5 |
| Aged control (N=9) | 398.8 ± 6.5† | 413.5 ± 13.5# |
| Aged trained (N=9) | 416.4 ± 5.0* | 373.8 ± 10.6 |
Values are means ± SE.
indicates significant (P ≤ 0.05) difference from young control, and young trained at pre-intervention.
indicates significant (P ≤ 0.05) difference from young trained at pre-intervention.
indicates significant (P ≤ 0.05) difference from all other groups at post-intervention.
indicates significant (P ≤ 0.05) difference from young trained, and aged trained at post-intervention.
All groups show significant (P ≤ 0.05) pre- to post-intervention difference.
NMJ morphology
Soleus (slow-twitch)
When examining pre-synaptic nerve terminal branching patterns of the slow-twitch NMJs that are predominant in the soleus, our initial ANOVA revealed significant training-related alterations in all but one of the variables quantified. That is, training increased branch number, total branch length, and branching complexity without affecting average length per branch. Post-hoc analysis, however, showed that this training-induced remodeling of nerve terminal branching is restricted to young animals. Indeed, not a single aspect of pre-synaptic structure was found to differ between aged control and aged trained rats. Nonetheless, significant main effects for aging were noted for total branch length, and average branch length with aged controls demonstrating greater lengths than those of young controls indicating that aging alone had resulted in expanded branch length.
In our analysis of the post-synaptic component of slow-twitch NMJs, it was established that significant interactive effects existed in perimeter length encompassing the total endplate (including stained and unstained regions), perimeter length around stained areas of endplates, total area of endplates (i.e., stained and unstained regions), as well as areas of stained clusters of ACh receptors. Post-hoc analysis demonstrated that training significantly enhanced total perimeter length, as well as total, and stained areas of post-synaptic endplates in young, but not aged animals. An atypical finding was that aged rats exhibited a training related decrease in stained perimeter length. However, it was also determined that aging alone impacted post-synaptic structure as aged controls exhibited longer total, and stained perimeter lengths than young controls, along with larger total and stained endplate areas than young controls. Yet, in determining pre- to post-synaptic coupling (i.e. terminal branch length to endplate area), and dispersion of ACh receptors at the endplate, no significant main effects for training or aging, or their interaction were detected. Results of the analysis of slow-twitch NMJs in the soleus are displayed in Table 2.
Table 2.
Effects of age and training on slow-twitch neuromuscular junctions of soleus muscle.
| Pre-synaptic nerve terminal branching |
Young control (N=10) |
Young trained (N=10) |
Aged control (N=9) |
Aged trained (N=9) |
|---|---|---|---|---|
| Branch number | 5.0 ± 1.3 | 6.4 ± 1.2** | 4.9 ± 1.6 | 5.9 ± 1.4 |
| Total branch length (µm) | 109.9 ± 26.5 | 144.8 ± 31.0§ | 139.2 ± 38.3 | 139.4 ± 34.0 |
| Average branch length (µm) | 23.1 ± 3.8 | 23.6 ± 4.6 | 29.2 ± 4.4‡ | 23.8 ± 4.9 |
| Branching complexity | 5.6 ± 2.1 | 10.2 ± 3.6§ | 7.8 ± 5.0 | 9.3 ± 4.5 |
| Post-synaptic endplate structure | ||||
| Total perimeter (µm) | 107.9 ± 17.4 | 132.1 ± 18.0§ | 129.7 ± 18.9§ | 116.5 ± 20.1 |
| Stained perimeter (µm) | 172.7 ± 79.1 | 223.1 ± 89.1 | 246.9 ± 73.6# | 179.4 ± 87.5 |
| Total area (µm2) | 566.9 ± 196.8 | 796.8 ± 239.9§ | 856.0 ± 227.7§ | 685.6 ± 233.3 |
| Stained area (µm2) | 473.8 ± 158.3 | 649.3 ± 151.4§ | 697.0 ± 215.6§ | 571.0 ± 163.2 |
| Dispersion (%) | 83.9 ± 9.6 | 84.7 ± 8.8 | 79.6 ± 8.2 | 84.7 ± 10.4 |
| Pre- to post-synaptic coupling | 4.4 ± 0.3 | 4.6 ± 0.2 | 5.0 ± 0.2 | 4.3 ± 0.5 |
Values are means ± SE.
indicates significant difference (P ≤ 0.05) from Young control, and Aged control.
indicates significant difference (P ≤ 0.05) from Young control.
indicates significant (P ≤ 0.05) difference from all other groups.
indicates significant (P ≤ 0.05) difference from Young control, and Aged trained.
Branching complexity = branch number × total branch length/100. Dispersion = stained area/total area. Pre- to post-synaptic coupling = endplate stained area/total nerve terminal branch length.
Soleus (fast-twitch)
In our analysis of the pre-synaptic morphology of NMJs residing on the small number of fast-twitch muscle fibers in the soleus, a main effect for age was detected in total and average branch length. Post-hoc results indicated that the total length of branching was smaller in aged controls than young control and young trained groups. In contrast, the effect of aging on average branch length existed only between the two trained groups (young > aged). The only significant effect for treatment was identified for branch number where training increased that variable in aged, but not young rats. Branching complexity was not affected by either training, or aging in fast-twitch NMJs of the soleus.
Morphometeric quantification of post-synaptic endplates of fast-twitch NMJs of the soleus indicated that significant effects of age, but not treatment, were apparent. More specifically, perimeter length around the total endplate, as well as perimeter length around stained clusters of ACh receptors, were both greater in young compared to aged, although post-hoc procedures failed to detect differences between any two of the four experimental groups. And in both total and stained endplate areas, there were significant effects of age (young > aged) with post-hoc results revealing that aged controls displayed smaller areas than young controls in total area, and smaller than both young control and young trained rats in stained area. Training failed to elicit any post-synaptic remodeling, and neither training, nor aging altered post-synaptic dispersion of ACh receptors, or pre- to post-synaptic coupling. Data pertaining to fast-twitch NMJs of the soleus are presented in Table 3.
Table 3.
Effects of age and training on fast-twitch neuromuscular junctions of soleus muscle.
| Pre-synaptic nerve terminal branching |
Young control (N=10) |
Young trained (N=10) |
Aged control (N=9) |
Aged trained (N=9) |
|---|---|---|---|---|
| Branch number | 4.1 ± 0.6 | 4.7 ± 0.7 | 3.0 ± 0.3# | 5.4 ± 0.7 |
| Total branch length (µm) | 97.8 ± 25.4 | 101.5 ± 8.8 | 47.4 ± 7.8* | 83.0 ± 11.4 |
| Average branch length (µm) | 23.0 ± 3.1 | 25.2 ± 5.1 | 16.6 ± 3.5† | 15.6 ± 1.5 |
| Branching complexity | 5.0 ± 2.4 | 5.0 ± 0.9 | 1.6 ± 0.2 | 5.0 ± 1.3 |
| Post-synaptic endplate structure | ||||
| Total perimeter (µm) | 129.6 ± 19.7 | 130.5 ± 16.7 | 89.9 ± 11.9 | 99.8 ± 17.4 |
| Stained perimeter (µm) | 253.5 ± 76.0 | 228.7 ± 44.9 | 128.8 ± 23.0 | 149.9 ± 39.1 |
| Total area (µm2) | 962.3 ± 267.5 | 898.4 ± 251.8 | 355.6 ± 76.1¶ | 488.9 ± 134.9 |
| Stained area (µm2) | 754.9 ± 188.4 | 659.8 ± 125.6 | 239.8 ± 63.1* | 406.6 ± 107.7 |
| Dispersion (%) | 82.2 ± 4.4 | 82.5 ± 6.8 | 73.5 ± 10.6 | 87.2 ± 5.1 |
| Pre- to post-synaptic coupling | 9.7 ± 2.2 | 6.3 ± 1.0 | 5.8 ± 1.3 | 4.9 ± 1.3 |
Values are means ± SE.
indicates significant difference (P ≤ 0.05) from Aged trained, and Young trained.
indicates significant difference (P ≤ 0.05) from Young control, and Young trained.
indicates significant (P ≤ 0.05) difference from Young trained.
indicates significant (P ≤ 0.05) difference from Young control, and trend (P=0.07) from Young trained.
Branching complexity = branch number × total branch length/100. Dispersion = stained area/total area. Pre- to post-synaptic coupling = endplate stained area/total nerve terminal branch length.
Plantaris (fast-twitch)
In distinction from the soleus, in the plantaris muscle it is NMJs of fast-twitch muscle fibers that are in the vast majority. Our quantification of these fast-twitch synapses began with pre-synaptic nerve terminal branching. Initial ANOVA results demonstrated that if a main effect was identified, it was for aging, not training, and that no significant interaction between those two main effects was evident. More specifically, it was found that in branch number, total branch length, and branching complexity measurements were higher in young compared to aged animals. Further, post-hoc results revealed that young controls displayed significantly greater branch number, total length of branching, and branching complexity than aged rats, both control and trained ones. But as with nerve terminals of the soleus, aging, and for that matter training, failed to impact average length per branch. With respect to post-synaptic endplate dimensions, there were no significant effects for age, training, or interaction between those variables in any of the parameters quantified. Moreover, pre- to post-synaptic coupling was unaffected by age, training, or their interaction. Data for fast-twitch NMJs of the plantaris can be viewed in Table 4.
Table 4.
Effects of age and training on fast-twitch neuromuscular junctions of plantaris muscle.
| Pre-synaptic nerve terminal branching |
Young control (N=10) |
Young trained (N=10) |
Aged control (N=9) |
Aged trained (N=9) |
|---|---|---|---|---|
| Branch number | 7.4 ± 0.6†† | 6.4 ± 0.4 | 6.1 ± 0.2 | 5.8 ± 0.6 |
| Total branch length (µm) | 145.2 ± 10.2†† | 137.4 ± 8.6 | 121.7 ± 3.8 | 120.2 ± 11.3 |
| Average branch length (µm) | 21.1 ± 1.0 | 22.4 ± 0.9 | 21.1 ± 0.6 | 20.9 ± 0.8 |
| Branching complexity | 12.5 ± 1.7†† | 9.8 ± 1.1 | 8.1 ± 0.5 | 8.0 ± 1.6 |
| Post-synaptic endplate structure | ||||
| Total perimeter (µm) | 143.6 ± 15.5 | 123.4 ± 14.2 | 133.9 ± 4.7 | 141.4 ± 9.8 |
| Stained perimeter (µm) | 309.5 ± 23.5 | 296.6 ± 31.5 | 264.8 ± 21.8 | 299.9 ± 28.0 |
| Total area (µm2) | 836.9 ± 84.4 | 711.1 ± 71.3 | 691.2 ± 40.2 | 789.4 ± 77.4 |
| Stained area (µm2) | 528.6 ± 55.6 | 434.1 ± 37.1 | 441.9 ± 25.0 | 525.1 ± 51.0 |
| Dispersion (%) | 64.5 ± 1.1 | 63.8 ± 3.1 | 65.3 ± 2.6 | 65.3 ± 2.7 |
| Pre- to post-synaptic coupling | 3.5 ± 0.3 | 3.5 ± 0.2 | 3.9 ± 0.3 | 4.6 ± 0.3* |
Values are means ± SE.
indicates significant difference (P ≤ 0.05) from Aged control, and Aged trained.
indicates significant difference (P ≤ 0.05) from Young control, and Young trained.
Branching complexity = branch number × total branch length/100. Dispersion = stained area/total area. Pre- to post-synaptic coupling = endplate stained area/total nerve terminal branch length.
Plantaris (slow-twitch)
NMJs located on the minority slow-twitch muscle fibers in the plantaris demonstrated a far greater sensitivity to aging and training than those of fast-twitch fibers of that muscle. Pre-synaptically, both age and training were noted to have significant effects on branch number, total branch length, and branching complexity. Post-hoc analysis determined that for branch number and total length, young control values were greater than those of aged animals, both control and trained, and that training led to a reduction in those pre-synaptic measures among aged, but not young rats. Although it was also found that branching complexity was greater among young controls than aged control and trained animals, it was confirmed that unlike branch number and total length, training diminished branching complexity not only among aged rats, but also young ones. Once again though, it was noted that average length per branch was resistant to the effects of aging, training, and even their interaction.
Post-synaptic components of the slow-twitch NMJs of the plantaris were also sensitive to aging and training. For example, perimeter length around the total endplate (i.e. stained and unstained regions) showed significant effects of age, treatment, and their interaction. That is, young controls were noted to have longer total perimeter lengths around endplates than all three other groups. This also reveals that while training significantly reduced total endplate perimeter length in young animals, no such training effect occurred among the aged, although aging alone had resulted in a decline in that parameter. Regarding perimeter length around stained receptor clusters only, it was established that an age effect was present with young controls having significantly higher values than both young and aged trained rats. In quantifying endplate areas – both total and stained only - young controls were significantly smaller than those of the three other groups, and training resulted in size decrements, but only among young animals, with aging alone having already decreased the size of endplates. Finally, while post-synaptic dispersion was once again impervious to the effects of age and training, pre- to post-synaptic coupling showed that a significant interactive effect led to a training-induced increase in the ratio of stained ACh receptor area to nerve terminal branch length, but this was only true among aged rats. Results of the analysis of slow-twitch NMJs of the plantaris are found in Table 5.
Table 5.
Effects of age and training on slow-twitch neuromuscular junctions of plantaris muscle.
| Pre-synaptic nerve terminal branching |
Young control (N=10) |
Young trained (N=10) |
Aged control (N=9) |
Aged trained (N=9) |
|---|---|---|---|---|
| Branch number | 7.8 ± 1.0 | 6.0 ± 0.3 | 5.7 ± 0.4 | 3.8 ± 0.7** |
| Total branch length (µm) | 169.9 ± 15.9‡‡ | 117.9 ± 8.0 | 138.5 ± 11.5 | 88.5 ± 14.1** |
| Average branch length (µm) | 23.0 ± 1.5 | 20.3 ± 1.5 | 24.7 ± 0.6 | 23.2 ± 0.7 |
| Branching complexity | 13.4 ± 2.1† | 7.7 ± 0.6 | 8.3 ± 2.1 | 3.9 ± 1.3** |
| Post-synaptic endplate structure | ||||
| Total perimeter (µm) | 179.3 ± 11.6‡ | 126.4 ± 4.8 | 135.1 ± 10.7 | 131.4 ± 6.3 |
| Stained perimeter (µm) | 367.5 ± 36.9# | 239.6 ± 16.4 | 270.2 ± 45.1 | 244.6 ± 50.9 |
| Total area (µm2) | 1081.1 ± 134. 1‡ | 713.2 ± 53.4 | 703.9 ± 92.1 | 635.5 ± 128.0 |
| Stained area (µm2) | 723.8 ± 83.9‡ | 498.2 ± 55.9 | 498.1 ± 52.1 | 415.1 ± 73.5 |
| Dispersion (%) | 68.2 ± 1.7 | 64.8 ± 3.1 | 72.1 ± 4.1 | 65.4 ± 5.5 |
| Pre- to post-synaptic coupling | 4.4 ± 0.4 | 4.3 ± 0.5 | 3.8 ± 0.3 | 8.1 ± 3.4‡ |
Values are means ± SE.
indicates significant difference (P ≤ 0.05) from Young control, and Aged control.
indicates significant difference (P ≤ 0.05) from Aged control.
indicates significant (P ≤ 0.05) difference from Young trained.
indicates significant (P ≤ 0.05) difference from all other groups.
indicates significant (P ≤ 0.05) difference from Young trained, and Aged trained.
Branching complexity = branch number × total branch length/100. Dispersion = stained area/total area. Pre- to post-synaptic coupling = endplate stained area/total nerve terminal branch length.
Muscle fiber morphology
Soleus
When examining the effects of aging and training on the size of muscle fibers irrespective of fiber type, the initial ANOVA revealed significant treatment and interactive effects. Follow-up post-hoc analysis indicated that the fibers of young control solei were significantly larger than those of all other experimental groups. Moreover, while it was determined that training led to a significant 20% decline in the size of muscle fibers in the young rats, it was discovered to have significantly increased fiber size by 14% in aged rats. This, however, must be viewed with the understanding that the average fiber size of aged controls was 19% smaller than that of young controls, thus presenting different reference points in determining the impact of training among the two age groups.
When exclusively quantifying type I fibers of the soleus, results mirrored those of when fiber types were pooled together. That is, significant treatment and interactive effects demonstrated that young control fibers were larger than those of the other three experimental groups (including aged controls), and that although training reduced fiber size in young animals by 23%, it expanded fiber size by 11% in aged rats. When examining type IIA fibers by themselves, no significant effects were detected for age, treatment, or their interaction. But type IIX fibers exhibited a significant treatment effect whereby training decreased fiber size in both young and aged rats by 21% and 17%, respectively.
Concerning fiber type composition of the soleus, ANOVA results of the percentage of type I fibers showed the presence of significant age and treatment effects. Post-hoc procedures indicated that training increased the percentage of type I fibers in young, but not aged rats. Among type IIA fibers there was a significant effect for treatment whereby training decreased the proportion of those fibers in young, but not aged solei. Finally, among type IIX fibers, no significant effects were identified. Data regarding muscle fiber profiles of soleus muscles are presented in Table 6.
Table 6.
Effects of age and training on muscle fiber profiles of soleus muscles.
| Cross-sectional area (µm2) | Young control (N=10) |
Young trained (N=10) |
Aged control (N=9) |
Aged trained (N=9) |
|---|---|---|---|---|
| Fiber types combined | 3022 ± 108‡ | 2420 ± 95@ | 2444 ± 160 | 2769 ± 161@ |
| Type I | 3164 ± 112‡ | 2436 ± 93@ | 2485 ± 165 | 2795 ± 160@ |
| Type IIA | 2277 ± 214 | 2397 ± 224 | 2306 ± 188 | 2086 ± 219 |
| Type IIX | 2659 ± 125 | 2106 ± 200@ | 2344 ± 171 | 1950 ± 146@ |
| Fiber type composition (%) | ||||
| Type I | 82.6 ± 2.4 | 89.6 ± 1.5** | 79.5 ± 3.3 | 83.1 ± 2.5 |
| Type IIA | 12.1 ± 2.4 | 7.6 ± 1.5** | 15.5 ± 3.0 | 11.4 ± 1.8 |
| Type IIX | 5.3 ± 0.5 | 2.8 ± 1.6 | 5.0 ± 1.5 | 5.5 ± 1.6 |
Values are means ± SE.
indicates significant difference (P ≤ 0.05) from all other groups.
indicates significant difference (P ≤ 0.05) from control group of the same age.
indicates significant (P ≤ 0.05) difference from Young control, and Aged control.
Plantaris
With fiber types collapsed together, a significant effect of treatment was detected for muscle fiber cross-sectional area. Post-hoc results indicated that training significantly decreased fiber size, but only among aged plantaris. And unlike in the soleus, this was true even though there was no difference between young controls, and aged controls. When examining the small number of type I fibers in the plantaris, no significant differences were found. In contrast, among the more plentiful type IIA fibers a significant effect of age was revealed whereby aged control fibers were smaller than young control, and young trained fibers. Finally, a significant treatment effect was detected among type IIX/B fibers, with post-hoc results showing that training was associated with a sharp decline in fiber size in young and aged rats (35% and 45%, respectively).
In examining our results on fiber type composition of plantaris muscles, the proportion of type I fibers was significantly affected by the interaction between age and treatment. More specifically, it was noted that although training increased the proportion of type I fibers in young plantaris muscles, it served to decrease the percentage of type I fibers in aged rats even though there was no pre-existing difference between young controls and aged controls. When focusing on type IIA fibers, a significant treatment effect was indicated, and post-hoc analysis showed that training increased the percentage of type IIA fibers both in young and aged animals. Along with this, it was documented that training resulted in a corresponding decrease in the percentage of type IIX/B fibers in young, as well as aged plantaris muscles. Results of our analysis of muscle fiber profiles of the plantaris can be viewed in Table 7.
Table 7.
Effects of age and training on muscle fiber profiles of plantaris muscles.
| Cross-sectional area (µm2) | Young control (N=10) |
Young trained (N=10) |
Aged control (N=9) |
Aged trained (N=9) |
|---|---|---|---|---|
| Fiber types combined | 2701 ± 165 | 2344 ± 128 | 2888 ± 182# | 1936 ± 113 |
| Type I | 862 ± 92 | 1006 ± 73 | 899 ± 117 | 821 ± 56 |
| Type IIA | 2398 ± 176 | 2400 ± 113 | 1827 ± 236* | 2108 ± 146 |
| Type IIX/B | 2659 ± 224 | 1730 ± 257** | 2932 ± 217 | 1612 ± 81** |
| Fiber type composition (%) | ||||
| Type I | 2.8 ± 0.4 | 4.0 ± 0.7 | 4.7 ± 0.5§ | 3.8 ± 0.4 |
| Type IIA | 16.2 ± 7.4 | 70.3 ± 7.0** | 11.2 ± 1.9 | 68.3 ± 4.0** |
| Type IIX/B | 81.0 ± 7.6 | 25.7 ± 7.1** | 84.1 ± 2.0 | 27.9 ± 3.0** |
Values are means ± SE.
indicates significant difference (P ≤ 0.05) from Young trained, and Aged trained.
indicates significant difference (P ≤ 0.05) from Young control, and Young trained.
indicates significant (P ≤ 0.05) difference from Young control, and Aged control.
indicates significant (P ≤ 0.05) difference from Young control.
Discussion
The principal objective of the present study was to determine whether aging modified the capacity of the neuromuscular system – particularly the NMJ – to respond to the stimulus of chronic endurance exercise training. It has been previously reported that among young adults, a multi-week regimen of treadmill running results in physiological adaptations (i.e. increased quantal content, or neurotransmitter released per impulse, decreased depression of quantal content during a train of stimuli), as well as morphological adaptations including enhanced nerve terminal branching, and increased post-synaptic endplate size (Deschenes et al., 1993; Dorlochter et al., 1991; Fahim, 1997). These training-induced adaptations of the NMJ were linked to improved endurance exercise performance (Andonian and Fahim, 1987; Dorlochter et al., 1991). This is an important observation because both longer and more intense exercise sessions provide greater health related benefits such as protection against coronary heart disease, stroke, bone weakness, and certain forms of cancer (Chodzko-Zajko et al., 2009). These are maladies that are keenly prevalent among the aged (Chodzko-Zajko et al., 2009; Chodzko-Zajko et al., 2009). With the growing number of aged people comprising the populations of the United States and other western nations, regular exercise training is currently viewed by health professionals as a vital prophylactic against, and modifier of, those life threatening conditions.
Numerous investigations have reported that aging by itself, results in remodeling of the NMJ (Deschenes et al., 2010; Rosenheimer and Smith, 1985; Valdez et al., 2010), but it is less clear how those age-related alterations influence the capacity of the NMJ to demonstrate the typical adaptations of that synapse to the stimulus of exercise training. Some insight into this question was obtained by Fahim (Fahim, 1997), who reported that aging attenuated the potential of the NMJ to respond to exercise both in terms of electrophysiology, and pre-synaptic nerve terminal arborization. These important findings, however, were gained by examining a single muscle – the gluteus – of rats, and without the distinction of responses exhibited by fast-twitch vs. slow-twitch NMJs from that single, large muscle with mixed fiber type. Here, we examined in greater detail the morphologic adaptations of fast- and slow-twitch NMJs in two different muscles – the soleus and plantaris – featuring disparate principal functions, and with different predominant expressions of muscle fiber type and NMJs. More directly, the main activity of the soleus is posture, and it is primarily composed of slow-twitch muscle fibers, with slow-twitch NMJs residing on them. In contrast, the plantaris plays little to no role in posture, but is heavily recruited during locomotor activity and quite the opposite of the soleus, it is fast-twitch muscle fibers, and their NMJs that overwhelmingly comprise that muscle. So by distinguishing between slow- and fast-twitch NMJs in what are predominantly slow- and fast-twitch muscles with distinct functions - and by doing so in both young adult and aged animals - we were able to reveal the more nuanced adaptations of the neuromuscular system to exercise training, its integrative nature, as well as how aging impacts the sensitivity of that system to endurance exercise.
On balance, our findings indicate that aging does, indeed, limit the capacity of NMJs to adapt to endurance training, if such adaptations are to occur. The most obvious case of this is found in the slow-twitch NMJs that are predominant in the soleus. In young adult rats, low intensity treadmill running evoked the same remodeling noted in previous investigations (Deschenes et al., 1993; Andonian and Fahim, 1988). In pre-synaptic measures, amplifications in nerve terminal branching were detected, such as increased branch number and total length resulting in greater branching complexity, even though average length per branch was unaffected. These were coupled with similar modifications at the post-synaptic endplate where training was associated with enlarged perimeter lengths, and endplate areas. This maintained normal, untrained pre- to post-synaptic ratios, as well as dispersion of ACh receptor clusters. Strikingly, however, aged rats submitted to the very same training regimen showed no evidence of training-induced reconfiguration of slow-twitch NMJs, both in pre- and post-synaptic components. Importantly, it was revealed that aging by itself had already resulted in the expansion of pre- and post-synaptic dimensions which, presumably, precluded the potential of training-induced modifications to occur in the slow-twitch NMJs of the soleus. Since it is known that the NMJ is a site of neuromuscular fatigue (Dorlochter et al., 1991; Desaulniers et al., 2001), it might be that the neuromuscular systems of aged individuals cannot optimally respond to chronic exercise training.
In contrast, our findings from the fast-twitch NMJs in that same soleus muscle provide just one example of training-induced NMJ adaptation (i.e. increased branch number), and this occurred only among aged animals, as opposed to young ones where slow-twitch NMJs were remodeled as a result of training. Moreover, although as with slow-twitch NMJs, aging alone was associated with pre- and post-synaptic remodeling, fast-twitch NMJs displayed significant decrements in size. Recall that among the soleus’ slow-twitch NMJs, aging elicited expansions in synaptic size. So although both the slow-twitch, and the fast-twitch NMJs of the soleus displayed age-related remodeling, the nature of such modifications differed greatly. It is of interest to note once again though that remodeling of NMJs in the soleus - in this instance fast-twitch ones - did not modulate average terminal branch length, or pre- to post-synaptic coupling. Apparently, there is effective communication between nerve terminals and their corresponding endplates on the muscle fiber’s surface during any synaptic reconfiguration, regardless of whether age, or exercise training is responsible. And although during an animals’ natural growth and development, changes in NMJ size can be attributed to similar, and proportional modifications in the size of the muscle fiber it resides on (Balice-Gordon and Lichtman, 1990; Wigston, 1989), this is not the case in the alterations in NMJ size identified here. Our muscle fiber analysis of the soleus showed that, as previously reported (Deschenes et al., 1993; Deschenes et al., 2006), endurance training reduced muscle fiber size in young adults even as NMJ size increased. It was also discovered that slow-twitch NMJs of the soleus grew in response to aging despite the fact that type I (slow-twitch) fibers had atrophied in aged solei. This is not surprising as it is already well established that aging typically results in NMJ expansion (Deschenes, 2011), and that sarcopenia, or the age-related remodeling of muscle results in muscle fiber atrophy (Deschenes, 2004; Narici and Maffulli, 2010). Yet it is of interest to note that adaptations of the NMJ to aging, and/or exercise training do not simply mimic those of their muscle fibers implying that different sets of mechanisms are activated within the muscle fiber compared to those at the fiber’s endplate region and associated nerve terminal.
Our investigation of the primarily fast-twitch plantaris muscle, whose main function differs from that of the soleus, also attests to the impressive sensitivity and specificity of the neuromuscular synapse to aging, exercise training, and the interaction of those two influences. For example, as in the synapses of the soleus, the fast-twitch NMJs of the plantaris display remodeling of pre-synaptic nerve terminals in aged animals. However, unlike the soleus where age affected both pre- and post-synaptic components of the NMJ, in the fast-twitch NMJs of the plantaris, no age- or training-related alterations in post-synaptic features were identified. And although aging alone decreased pre- and post-synaptic dimensions in the minority slow-twitch NMJs of the plantaris, nerve terminal branching, as well as endplate size, increased as a result of aging in the slow-twitch synapses of the soleus. Similarly, while exercise training modified pre- and post-synaptic structure among slow-twitch NMJs in both the soleus and plantaris, the direction of those modifications was polar opposite in those two muscles. More specifically, slow-twitch NMJs of young adult solei were increased in size by endurance training, while the dimensions of the slow-twitch NMJs of the plantaris were decreased by training. Further, in the plantaris endurance training modified slow-twitch NMJ structure in both young adult, and aged animals, though the effects of exercise training were evident only among the slow-twitch NMJs of young adult soleus muscles.
Presumably, the various adaptations observed in NMJs that corresponded to aging, and exercise training were brought about by changes in the expression of molecules (e.g. NCAM, NT-4, N-cadherin) that regulate the structure of synapses, including the NMJ, and can be secreted from pre- and post-synaptic sites. There is a bevy of these molecules, some of which have been shown to be sensitive to aging (Kobayashi et al., 1992; Deschenes and Wilson, 2003; Apel et al., 2010), while others are known to be sensitive to alterations in the degree of neuromuscular activity (Deschenes and Wilson, 2003; Funakoshi et al., 1995; Covault et al., 1986; Sanes et al., 1986). In the present investigation, we did not assess alterations in the secretion in any of these molecules. What can be ascertained from the findings reported here, however, is that there is perhaps a higher level of complexity than realized in the regulation of these synapse modifying molecules at the whole organism, or system levels. While much insight is gained by the studying synapses of a single type from a single tissue regarding synaptic plasticity and its control, the present study of NMJs located in functionally divergent muscles, and on different fiber types within those muscles revealed a remarkable level of neuromuscular coordination, specificity, and sensitivity in responding to the natural phenomenon of aging, as well as increased activity (i.e. exercise training). The present data demonstrate that it cannot be assumed that what occurs at one type of synapse in one organ or tissue type is necessarily replicated at all synapses in that system. Indeed, it was revealed here that quite opposite adaptations may be evident at different synaptic sites, and even within synapses of different fiber types within the same muscle. This coordination of disparate neuromuscular responses to differences in age and activity patterns, particularly of the NMJ, requires additional investigation so that we may gain deeper insight and understanding, particularly of the mechanisms that govern such specific remodeling of neural networking.
To more thoroughly appreciate the adaptability of the neuromuscular system to the effects of aging, and exercise training muscle fiber profiles were quantified in the same muscles used to examine NMJs. As previously reported, it was determined that in young adult soleus muscles, a regimen of endurance training led to a significant decline in fiber size (Deschenes et al., 1993; Deschenes et al., 2006). This was evident with fiber types pooled together, among the predominant type I fibers of the soleus, as well as the type IIX fibers. This training-induced decrement in size is typically viewed as a positive adaptation as it decreases the distance that oxygen and carbon dioxide must passively diffuse across the fiber when entering, or leaving it, respectively (Hoppeler and Vogt, 2001; Hoppeler et al., 1990). This loss of fiber size associated with training is in sharp distinction from what occurred in aged animals where trained aged rats had larger muscle fibers than untrained aged rats. This perhaps can be attributed to the fact that aging alone had decreased fiber size creating potential for fiber hypertrophy, even in response to endurance training which does not commonly elicit fiber hypertrophy. Indeed, it has been reported previously that among aged humans endurance training results in muscle fiber hypertrophy (Coggan et al., 1992), and our laboratory has recently found that as little as two weeks of endurance training increases muscle fiber size in the soleus muscle of aged rats (Deschenes et al., unpublished observations).
The only difference in soleus fiber type composition among the four experimental groups was that the young adult trained group had a higher percentage of type I fibers, and lower percentage of type IIA fibers than the other three groups, again indicating that aging may limit training-induced neuromuscular plasticity. Previous investigations have also established that endurance training increases the percentage of type I fibers in the soleus, while the proportion of type II fibers is concomitantly reduced (Stebbins et al., 1985; Wernig et al., 1990).
With respect to the plantaris, it was found that endurance training evoked a significant decline in muscle fiber size (types combined, and the predominant type IIX/B fibers) in both young adult, and aged animals. This difference from the adaptations documented in the soleus – where only young adults experienced training-induced size decrements – is probably related to the fact that in the plantaris there was no reduction in fiber size associated with aging by itself. Thus, when related to untrained values, endurance training resulted in the same atrophic responses in both young adult, and aged plantaris muscles. Training-related alterations in the fiber type composition of the plantaris also did not demonstrate age specificity. Indeed, both young adult, and aged plantaris muscles displayed significant increases in the proportion of type IIA fibers that were coupled with significant decreases in the percentage of type IIX/B fibers. These muscle fiber profile data also speak to the impressive sensitivity and specificity of the neuromuscular system to the effects of aging, and exercise training. That is, while the training-induced adaptations of the muscle fibers of the soleus differed between younger and older animals, the fibers of the plantaris muscles of those same young adult, and aged rats exhibited similar remodeling as a consequence of exercise training.
Conclusions
In summary, the main objective of this study was to assess whether aging impacted the capacity of the neuromuscular system, particularly the NMJ, to adapt to endurance exercise training, and whether this effect would be specific to muscles and/or to certain muscle fiber types within different muscles. Indeed, it is the fact that we were able to examine slow- vs. fast-twitch NMJs within the same muscle, whether it was the primarily slow-twitch soleus, or the predominantly fast-twitch plantaris, that makes this study unique and informative. Based on the findings presented here, it does appear that, in general, aging tempers the potential of the neuromuscular system to adapt to the stimulus exercise training. Because the NMJ is a site of neuromuscular fatigue, its impaired ability to adapt to exercise training among the aged may limit the volume and intensity of exercise sessions, and by extension, the health benefits derived from a program of regular exercise among those who are in most need of them. This is important applied information, but in gleaning this information by examining synaptic responses in slow- and fast-twitch NMJs to aging, and exercise training both in the postural soleus muscle, and the mainly locomotor plantaris, the complexity of the regulation of the neuromuscular system was brought to light. It was noted that the synaptic remodeling brought about by aging, and exercise is characterized by an impressive degree of specificity and sensitivity, not only among different muscles throughout the body, but also among different fiber types within the same muscle. What remains to be more fully illuminated are the mechanisms, by way of differential expression of synapse regulating molecules, used by the neuromuscular system to coordinate such diverse and specific adaptations.
Highlights.
-
-
We have examined whether aging affects exercise-induced remodeling of the neuromuscular junction (NMJ)
-
-
Alone, aging was found to result in significant morphological alterations of the NMJ
-
-
It was also determined that aged and young adult rats display disparate remodeling of the NMJ following 10 weeks of treadmill running
-
-
Most significantly, our data show that exercise related remodeling of the NMJ differs between the soleus and plantaris muscles, and it is fiber type specific within those muscles
Acknowledgements
This work was supported by grants from the National Institutes of Health (grant number R15 AG17440), and the Howard Hughes Medical Institute through a grant to the Undergraduate Biological Sciences Education Program at The College of William & Mary.
Abbreviations
- ACh
acetylcholine
- BSA
bovine serum albumin
- BTX
bungarotoxin
- NMJ
neuromuscular junction
- PBS
phosphate buffered saline
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderton BH, Breinburg D, Downes MJ, Green PJ, Tomlinson BE, Ulrich J, Wood JN, Kahn J. Monoclonal antibodies show that neurofibrillary tangles and neurofilaments share antigenic determinants. Nature. 1982;298:84–86. doi: 10.1038/298084a0. [DOI] [PubMed] [Google Scholar]
- Andonian MH, Fahim MA. Endurance exercise alters the morphology of fast- and slow-twitch rat neuromuscular junctions. Int J Sports Med. 1988;9:218–223. doi: 10.1055/s-2007-1025009. [DOI] [PubMed] [Google Scholar]
- Andonian MH, Fahim MA. Effects of endurance exercise on the morphology of mouse neuromuscular junctions during ageing. J Neurocytol. 1987;16:589–599. doi: 10.1007/BF01637652. [DOI] [PubMed] [Google Scholar]
- Apel PJ, Ma J, Callahan M, Northam CN, Alton TB, Sonntag WE, Li Z. Effect of locally delivered IGF-1 on nerve regeneration during aging: an experimental study in rats. Muscle Nerve. 2010;41:335–341. doi: 10.1002/mus.21485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias E. United States life tables, 2003. Natl Vital Stat Rep. 2006;54:1–40. [PubMed] [Google Scholar]
- Balice-Gordon RJ, Lichtman JW. In vivo visualization of the growth of pre- and postsynaptic elements of neuromuscular junctions in the mouse. J Neurosci. 1990;10:894–908. doi: 10.1523/JNEUROSCI.10-03-00894.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belluardo N, Westerblad H, Mudo G, Casabona A, Bruton J, Caniglia G, Pastoris O, Grassi F, Ibanez CF. Neuromuscular junction disassembly and muscle fatigue in mice lacking neurotrophin-4. Mol Cell Neurosci. 2001;18:56–67. doi: 10.1006/mcne.2001.1001. [DOI] [PubMed] [Google Scholar]
- Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, Skinner JS. American College of Sports Medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530. doi: 10.1249/MSS.0b013e3181a0c95c. [DOI] [PubMed] [Google Scholar]
- Coggan AR, Spina RJ, King DS, Rogers MA, Brown M, Nemeth PM, Holloszy JO. Skeletal muscle adaptations to endurance training in 60- to 70-yr-old men and women. J Appl Physiol. 1992;72:1780–1786. doi: 10.1152/jappl.1992.72.5.1780. [DOI] [PubMed] [Google Scholar]
- Covault J, Merlie JP, Goridis C, Sanes JR. Molecular forms of N-CAM and its RNA in developing and denervated skeletal muscle. J Cell Biol. 1986;102:731–739. doi: 10.1083/jcb.102.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Desaulniers P, Lavoie PA, Gardiner PF. Habitual exercise enhances neuromuscular transmission efficacy of rat soleus muscle in situ. J Appl Physiol. 2001;90:1041–1048. doi: 10.1152/jappl.2001.90.3.1041. [DOI] [PubMed] [Google Scholar]
- Deschenes MR. Effects of aging on muscle fiber type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- Deschenes MR. Motor unit and neuromuscular junction remodeling with aging. Curr Aging Sci. 2011 doi: 10.2174/1874609811104030209. Epub ahead of print PMID 21529328. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Maresh CM, Crivello JF, Armstrong LE, Kraemer WJ, Covault J. The effects of exercise training of different intensities on neuromuscular junction morphology. J Neurocytol. 1993;22:603–615. doi: 10.1007/BF01181487. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Roby MA, Eason MK, Harris MB. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp Gerontol. 2010;45:389–393. doi: 10.1016/j.exger.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR, Tenny KA, Wilson MH. Increased and decreased activity elicits specific morphological adaptations of the neuromuscular junction. Neuroscience. 2006;137:1277–1283. doi: 10.1016/j.neuroscience.2005.10.042. [DOI] [PubMed] [Google Scholar]
- Deschenes MR, Wilson MH. Age-related differences in synaptic plasticity following muscle unloading. J Neurobiol. 2003;57:246–256. doi: 10.1002/neu.10271. [DOI] [PubMed] [Google Scholar]
- Dorlochter M, Irintchev A, Brinkers M, Wernig A. Effects of enhanced activity on synaptic transmission in mouse extensor digitorum longus muscle. J Physiol. 1991;436:283–292. doi: 10.1113/jphysiol.1991.sp018550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Abraham WM, Terjung RL. Influence of exercise intensity and duration on biochemical adaptations in skeletal muscle. J Appl Physiol. 1982;53:844–850. doi: 10.1152/jappl.1982.53.4.844. [DOI] [PubMed] [Google Scholar]
- Fahim MA. Endurance exercise modulates neuromuscular junction of C57BL/6NNia aging mice. J Appl Physiol. 1997;83:59–66. doi: 10.1152/jappl.1997.83.1.59. [DOI] [PubMed] [Google Scholar]
- Funakoshi H, Belluardo N, Arenas E, Yamamoto Y, Casabona A, Persson H, Ibanez CF. Muscle-derived neurotrophin-4 as an activity-dependent trophic signal for adult motor neurons. Science. 1995;268:1495–1499. doi: 10.1126/science.7770776. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Howald H, Cerretelli P. Human muscle structure after exposure to extreme altitude. Experientia. 1990;46:1185–1187. doi: 10.1007/BF01936933. [DOI] [PubMed] [Google Scholar]
- Hoppeler H, Vogt M. Muscle tissue adaptations to hypoxia. J Exp Biol. 2001;204:3133–3139. doi: 10.1242/jeb.204.18.3133. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Robbins N, Rutishauser U. Neural cell adhesion molecule in aged mouse muscle. Neuroscience. 1992;48:237–248. doi: 10.1016/0306-4522(92)90352-3. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Armstrong RB. Muscular blood flow distribution patterns as a function of running speed in rats. Am J Physiol. 1982;243:H296–H306. doi: 10.1152/ajpheart.1982.243.2.H296. [DOI] [PubMed] [Google Scholar]
- Liu CK, Fielding RA. Exercise as an intervention for frailty. Clin Geriatr Med. 2011;27:101–110. doi: 10.1016/j.cger.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull. 2010;95:139–150. doi: 10.1093/bmb/ldq008. [DOI] [PubMed] [Google Scholar]
- Nemeth P, Pette D. Succinate dehydrogenase activity in fibres classified by myosin ATPase in three hind limb muscles of rat. J Physiol. 1981;320:73–80. doi: 10.1113/jphysiol.1981.sp013935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panenic R, Gisiger V, Gardiner PF. Fatigability of rat hindlimb muscles after acute irreversible acetylcholinesterase inhibition. J Appl Physiol. 1999;87:1455–1462. doi: 10.1152/jappl.1999.87.4.1455. [DOI] [PubMed] [Google Scholar]
- Pearson J, Sabarra A. A method for obtaining longitudinal cryostat sections of living muscle without contraction artifacts. Stain Technol. 1974;49:143–146. doi: 10.3109/10520297409116965. [DOI] [PubMed] [Google Scholar]
- Prakash YS, Sieck GC. Age-related remodeling of neuromuscular junctions on type-identified diaphragm fibers. Muscle Nerve. 1998;21:887–895. doi: 10.1002/(sici)1097-4598(199807)21:7<887::aid-mus6>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Rosenheimer JL, Smith DO. Differential changes in the end-plate architecture of functionally diverse muscles during aging. J Neurophysiol. 1985;53:1567–1581. doi: 10.1152/jn.1985.53.6.1567. [DOI] [PubMed] [Google Scholar]
- Roy RR, Hirota WK, Kuehl M, Edgerton VR. Recruitment patterns in the rat hindlimb muscle during swimming. Brain Res. 1985;337:175–178. doi: 10.1016/0006-8993(85)91627-0. [DOI] [PubMed] [Google Scholar]
- Ryan AS. Exercise in aging: its important role in mortality, obesity and insulin resistance. Aging health. 2010;6:551–563. doi: 10.2217/ahe.10.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Schachner M, Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin, and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle. J Cell Biol. 1986;102:420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherder EJ, Bogen T, Eggermont LH, Hamers JP, Swaab DF. The more physical inactivity, the more agitation in dementia. Int Psychogeriatr. 2010;22:1203–1208. doi: 10.1017/S1041610210001493. [DOI] [PubMed] [Google Scholar]
- Sieck GC, Prakash YS. Fatigue at the neuromuscular junction. Branch point vs. presynaptic vs. postsynaptic mechanisms. Adv Exp Med Biol. 1995;384:83–100. [PubMed] [Google Scholar]
- Stebbins CL, Schultz E, Smith RT, Smith EL. Effects of chronic exercise during aging on muscle and end-plate morphology in rats. J Appl Physiol. 1985;58:45–51. doi: 10.1152/jappl.1985.58.1.45. [DOI] [PubMed] [Google Scholar]
- Tomas J, Fenoll R, Mayayo E, Santafe M. Branching pattern of the motor nerve endings in a skeletal muscle of the adult rat. J Anat. 1990;168:123–135. [PMC free article] [PubMed] [Google Scholar]
- Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci. 1999;54:B492–B501. doi: 10.1093/gerona/54.11.b492. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau (a) International database. Table 094. Midyear population, by age and sex. Available at http://www.census.gov/population/www/projections/natdet-D1A.html.
- U.S. Census Bureau (b) State and national population projections. Available at http://www.census.gov/population/www/projections/popproj.html.
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernig A, Irintchev A, Weisshaupt P. Muscle injury, cross-sectional area and fibre type distribution in mouse soleus after intermittent wheel-running. J Physiol. 1990;428:639–652. doi: 10.1113/jphysiol.1990.sp018232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigston DJ. Remodeling of neuromuscular junctions in adult mouse soleus. J Neurosci. 1989;9:639–647. doi: 10.1523/JNEUROSCI.09-02-00639.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]