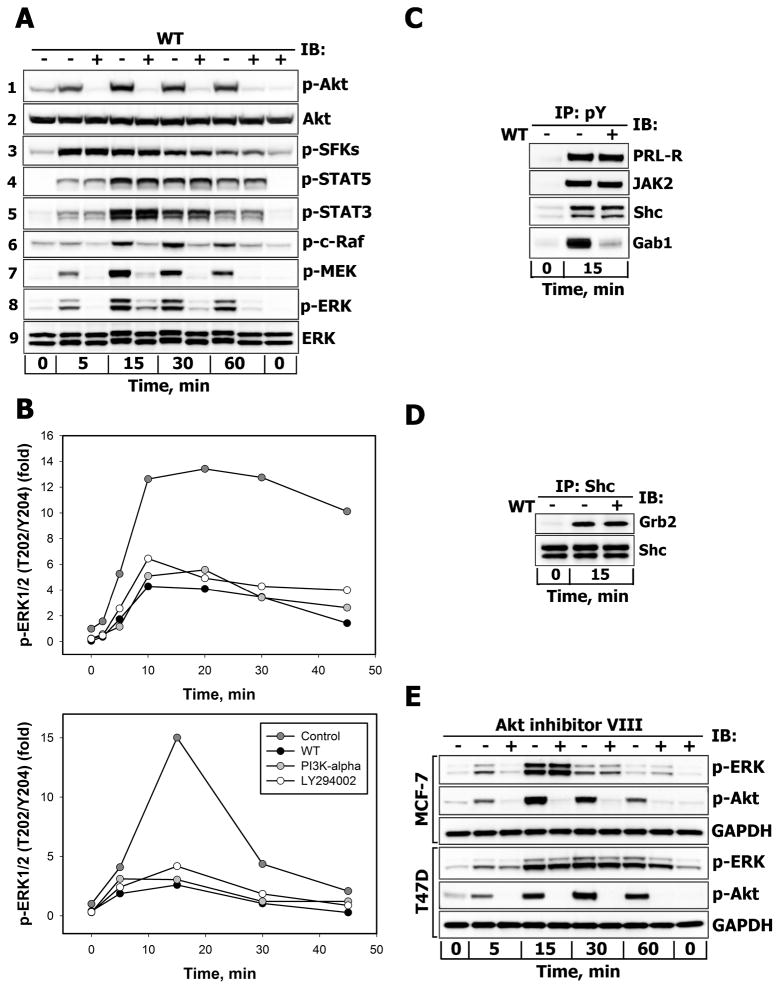
Figure 5.
A. Effects of PI3-kinase inhibition on the activation of downstream effectors of PRL-R Serum-starved T47D cells were either left untreated (−) or were treated (+) with wortmannin (WT, 200 nM, 30 min) before stimulation with 10 nM PRL for the indicated time intervals. Phosphorylated forms of Akt (Ser473), SFKs (Tyr416), STAT5 (Tyr694), STAT3 (Tyr705), c-Raf (Ser338), MEK1/2 (Ser217/Ser221) and ERK1/2 (Thr202/Tyr204) proteins were detected by IB of the TCL with Abs against respective antigens. Total Akt and ERK1/2 protein levels were detected with anti-Akt1 or anti-ERK1/2 Abs, respectively, and served as protein loading controls. Representative blots are shown (n=3). B. Effects of structurally different inhibitors of PI3-kinase on PRL-induced activation of ERK1/2. Serum-starved T47D (upper panel) and MCF-7 (lower panel) cells were either left untreated (control, dark grey circles) or were preincubated with WT (200 nM, black circles), PI3K-α inhibitor 2 (2 μM, grey circles) or LY 294002 (25 μM, white circles) for 30 min before stimulation with 10 nM PRL for the indicated time intervals. Both total and phosphorylated forms of ERK1/2 were detected by IB of the TCL with anti-ERK1/2 or anti-phospho-ERK1/2 (Thr202/Tyr204) Abs, respectively. The ratio of phospho-ERK1/2:total ERK1/2 at each time point is expressed as fold changes over basal levels. Quantitation of represenatative blots is shown (n=3). C. Effects of PI3-kinase inhibition on tyrosine phosphorylation levels of PRL-R, JAK2, Shc and Gab proteins. Tyrosine phosphorylated proteins were immunoprecipitated (IP) from TCL of unstimulated or PRL-stimulated (10 nM, 15 min) and WT-treated (+) (200 nM, 30 min) or untreated (−) T47D cells and probed for proteins indicated on the right. D. Effects of PI3-kinase inhibition on Shc association with Grb2. Shc proteins were immunoprecipitated (IP) from TCL of unstimulated or PRL-stimulated (10 nM, 15 min) and WT-treated (+) (200 nM, 30 min) or untreated (−) T47D cells and probed with anti-Grb2 or anti-Shc Abs. E. Effects of Akt inhibition on PRL-induced activation of ERK1/2. Serum-starved MCF-7 or T47D cells were either left untreated (−) or were treated (+) with Akt1/2/3 inhibitor (Akt-VIII, 10 μM, 30 min) before stimulation with 10 nM PRL for the indicated time intervals. Phosphorylated forms of Akt (Ser473) and ERK1/2 (Thr202/Tyr204) proteins were detected by IB of the TCL with Abs against respective antigens. GAPDH levels served as protein loading control. Representative blots are shown (n=3).