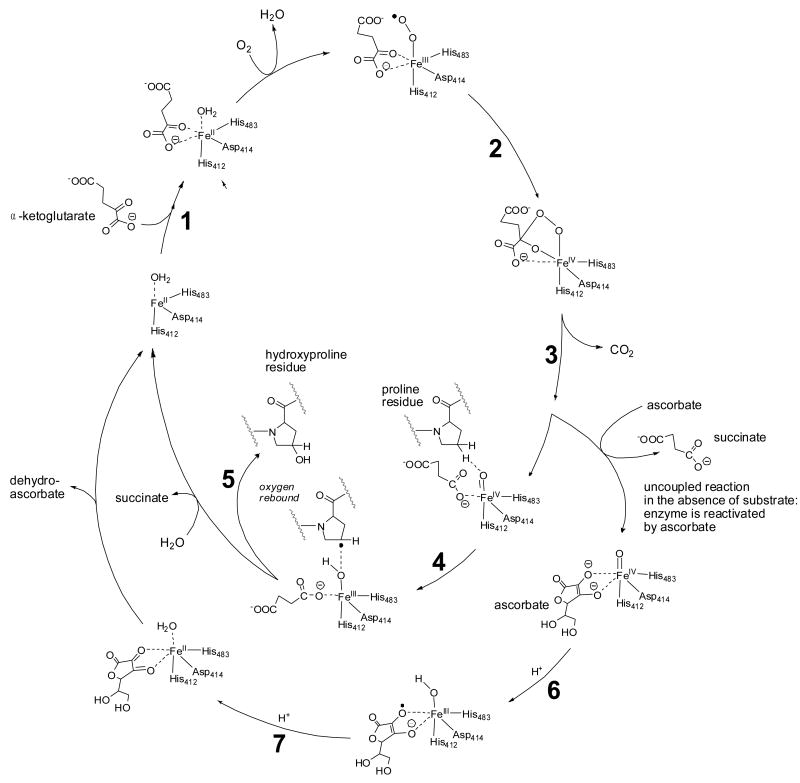
Fig. 1.
Vitamin C acts as a cofactor for prolyl 4-hydroxylase. The starting point for the catalytic cycle is where the co-substrate, α-ketoglutaric acid, coordinates with the enzyme-bound FeII (step 1). Activation of molecular oxygen (2) and subsequent decarboxylation of α-ketoglutaric acid (3) leads to the formation of the highly energetic FeIV=O reagent that hydroxylates proline residues in procollagen (steps 4 and 5, inner catalytic cycle) [217-219]. If decarboxylation of α-ketoglutaric acid and subsequent formation of the FeIV=O species takes place in the absence of a substrate molecule (proline residue), the FeIV=O species will oxidize a molecule of ascorbic acid in order to regain activity (steps 6 and 7, outer cycle). Thus, ascorbic acid is consumed stoichiometrically in the uncoupled reaction but is not consumed when substrate is available for oxidation. The iron is bound to prolyl 4-hydroxylase through interactions with amino acid residues, His 412, Asp414, and His 483 [10]. The co-substrate, α-ketoglutaric acid, binds to the enzyme-bound iron through two coordination sites as shown. These coordination sites can be occupied by ascorbate upon departure of succinate from the FeIV=O species in the uncoupled reaction (outer cycle).