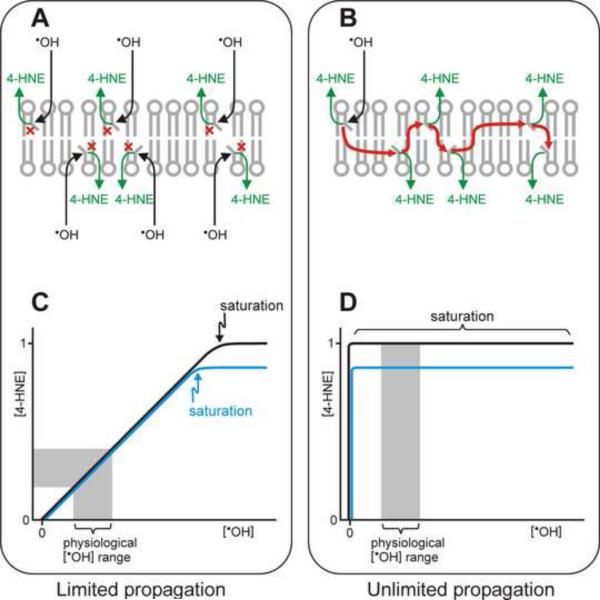
Fig. 5.
Relationship between the concentrations of initial ROS and resulting lipid peroxidation products. Panels A and B: Two theoretical extreme cases are schematically depicted in which the lipid peroxidation chain reaction terminates after a single cycle (A), or does not terminate until all PUFA substrate is used up (B). In the first case (panel A), a hydroxyl radical ˙OH attacks a PUFA (denoted by a bent fatty acyl chain in a membrane phospholipid), resulting in the formation of a lipid peroxidation product, for simplicity denoted as 4-HNE. The chain reaction is then immediately terminated (shown by the red “×” symbol). Thus, the formation of each 4-HNE molecule requires a separate attack on a PUFA by ˙OH. At the other extreme (panel B), a single initiation event starts a chain reaction (red arrows) that continues indefinitely, generating a 4-HNE molecule from each PUFA. Panels C and D depict graphically the two idealized relationships (corresponding to A and B, respectively) between the initiating ˙OH concentration and the amount of resulting 4-HNE. The black and blue lines in panels C and D denote, respectively, a higher and lower content of peroxidizable PUFA in the membrane. For rapidly terminating chain reactions (panel C, depicting results of the mechanism shown in panel A), there is a stoichiometric relationship between [˙OH] and [4-HNE] over a wide range of [˙OH], including a range that is physiologically normal (grey zone in panel C). In this range, the amount of 4-HNE formed is sensitive to the level of oxidative stress but not to the PUFA amount in the membrane, except for a very intensive oxidative burst that would deplete all PUFAs simultaneously (saturation point in panel C). Only under conditions of very high oxidative stress does the PUFA amount play a role; for example, less peroxidizable PUFA would lead to less 4-HNE formed under saturating [˙OH] (blue line). In the case of unlimited propagation of the chain reaction (panel D, illustrating results from the mechanism shown in panel B), a very small amount of ˙OH initiates a reaction that uses up all available PUFA and produces a maximal amount of 4-HNE. Thus, under these conditions, the formation of 4-HNE is a function of the content of peroxidizable PUFA in the membrane (black versus blue line in panel D) but is independent on the ˙OH concentration, including the physiological [˙OH] range (grey zone). Note that the two depicted situations are idealized extremes; an actual membrane may exhibit an intermediate behavior.