Abstract
Correct classification of non-alcoholic steatohepatitis (NASH) liver biopsies is of critical importance and relies on correct orientation to microscopic liver architecture. Centrizonal arteries can cause central zones to be mistaken for portal tracts, especially in the setting of centrizonal ductular reaction, and result in either missed diagnosis or inaccurate staging of NASH. 100 randomly selected biopsies from NASH Clinical Research Network participants (2/05-8/06, fibrosis stage >1a,) were evaluated for arteries and CD34 positive microvessels in the centrizonal region. Prevalence of both centrizonal arteries and CD34 positive microvessels was graded as 0 (none in central zones), 1 (1-2 central zones with vessels), 2 (< 50% of central zones with vessels), or 3 (≥ 50% of central zones with vessels). Centrizonal arteries and CD34 positive microvessels were present in 40 and 100 cases (40% and 100%), respectively. Arteries and CD34 positive microvessels were more commonly found in central zones in biopsies with greater degrees of fibrosis; 62% with arteries in stage 3-4 vs. 21% in stage 1-2 and 70% with microvessels in stage 3-4 vs. 25% in stage 1-2, with increased prevalence of both centrizonal arteries and CD34 positive microvessels correlating directly with fibrosis stage (p<0.001). Ductular reaction was a common finding (55%) in patients with central zone arteries. The presence of an aberrant centrizonal artery must be recognized to allow for correct orientation to liver architecture in NASH and, together with the finding of increased CD34 positive microvessel formation in higher stage fibrosis, suggests a possible association between neoangiogenesis and NASH progression to cirrhosis.
INTRODUCTION
Nonalcoholic steatohepatitis (NASH) is among the most common types of liver disease in the United States and Western Europe, and the incidence is rising in the developed world (4, 7, 9, 14, 21, 26). This increase in prevalence of NASH is likely due to the rising frequency of obesity and resultant metabolic syndrome, an entity that encompasses the problem of insulin resistance and the serious clinical sequelae of type 2 diabetes, hypertension, dyslipidemia and cardiovascular disease (14, 21, 26). The pathologist plays an important role in the recognition and appropriate treatment of steatohepatitis by examining the liver biopsy for a pattern of histologic findings typical for this lesion (12). In particular, centrizonal hepatocyte injury and fibrosis are established markers of alcoholic and nonalcoholic steatohepatitis, so recognition by the pathologist that injury has occurred in the central zone (zone 3) is of key importance (3, 12), and the correct identification of portal tracts and central veins, or their remnants, are vital to this process.
We have noted in our review of liver biopsies from patients with nonalcoholic steatohepatitis (NASH) that the region of the centrizonal scar may contain arteries (10). This finding has led to the misinterpretation of a centrizonal region as a portal zone by pathologists (personal observation of the authors and NASH Clinical Research Network pathologists), resulting either in an equivocal diagnosis of NASH or the exclusion of NASH as a possible diagnosis and potentially also to the inaccurate staging of NASH. This study examines the extent of this problem by review of liver biopsies from a collection of patients with well-documented NASH, with varying degrees of fibrosis, and utilizes CD34 immunohistochemical staining to evaluate for microvessel formation in this region.
MATERIALS AND METHODS
One hundred patients enrolled in the NASH Clinical Research Network (NASH CRN) from the time period of February 2005 through August 2006 were selected randomly from the NASH CRN database for this study, thus ensuring that this group of patients had a confirmed clinical and pathological diagnosis of NASH. Patients were adults and the age range was 21-73 years (except one 13 year old child with features of typical NASH). No patients with type 2 NASH (which is characterized by portal fibrosis and is found largely in children) were included (23). All selected biopsies had a fibrosis stage of greater than 1a, as determined by the NASH CRN system of grading and staging liver biopsies (12). The NASH CRN system separates early stage fibrosis into: 1a (Mild zone 3 perisinusoidal fibrosis, only seen on trichrome stain), 1b (Moderate zone 3 perisinusoidal fibrosis), and 1c (periportal fibrosis only). Our study did include two cases of adults with stage 1c fibrosis, the remainder of stage 1 cases were 1b. Hematoxylin-eosin (H&E) and trichrome stains from the enrollment liver biopsy were reviewed on each patient by Drs. Ferrell and Gill (LDF & RMG) for the presence of an artery in the centrizonal scar and graded on a scale of 0 to 3 (as described in Table 1). CD34 immunostained sections from each biopsy were then reviewed and scored for the presence of microvessels by LDF & RMG and graded on a similar scale of 0 to 3 (as described in Table 2). Microvessels (27, 28) were defined as distinct CD34 positive endothelial cell clusters, typically with a vessel lumen, and not clearly representing a component of diffuse sinusoidal capillarization (i.e. diffuse sinusoidal expression of CD34). All slides had at least five central zones and had been deemed adequate for assigning diagnosis and stage of NASH on central review by the NASH CRN Pathology Committee.
Table 1. Non-alcoholic steatohepatitis fibrosis stage versus prevalence of centrizonal arteries (grade)*.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Total (%) | |
|---|---|---|---|---|---|
| Stage 1b/1c | 17 | 2 | 0 | 0 | 2/19 (11%) |
| Stage 2 | 25 | 7 | 1 | 1 | 9/34 (27%) |
| Stage 3 | 15 | 8 | 10 | 3 | 21/36 (58%) |
| Stage 4 | 3 | 1 | 2 | 5 | 8/11 (73%) |
| Total | 60 | 18 | 13 | 9 | 40/100 (40%) |
Artery grades: 0 = no central zones with artery, 1 = 1-2 central zones with artery/biopsy, 2 = > 2 and < 50% of central zones with artery, 3 = ≥ 50% of central zones with artery. Definitions of stages: 1b = Centrizonal fibrosis only, without the use of trichrome stain (i.e. readily discernible on H&E stain), 1c = Periportal fibrosis only, 2 = Centrizonal and periportal, 3 = Bridging fibrosis, 4 = Cirrhosis.
P < 0.001 using univariate ordinal logistic regression.
Table 2. Non-alcoholic steatohepatitis fibrosis stage versus prevalence of centrizonal microvessels (grade)*.
| Grade 0 | Grade 1 | Grade 2 | Grade 3 | Total (%) | |
|---|---|---|---|---|---|
| Stage 1b/1c | 8 | 6 | 5 | 0 | 11/19 (58%) |
| Stage 2 | 13 | 13 | 6 | 2 | 21/34 (62%) |
| Stage 3 | 4 | 8 | 20 | 4 | 32/36 (89%) |
| Stage 4 | 2 | 0 | 3 | 6 | 9/11 (82%) |
| Total | 27 | 27 | 34 | 12 | 73/100 (73%) |
Microvessel (MV) grades: 0 = no central zones with MV, 1 = 1-2 central zones with MV/biopsy, 2 = > 2 and < 50% of central zones with MV, 3 = ≥ 50% of central zones with MV. Definitions of stages: 1b = Centrizonal fibrosis only, without the use of trichrome stain (i.e. readily discernible on H&E stain), 1c = Periportal fibrosis only, 2 = Centrizonal and periportal, 3 = Bridging fibrosis, 4 = Cirrhosis.
P < 0.001 using univariate ordinal logistic regression.
Histologic features, scored centrally by NASH CRN pathologists (including steatosis grade, steatosis location, lobular inflammation, ballooned hepatocytes, and fibrosis stage) were obtained from the database. The NAFLD Activity Score (NAS), defined as the sum of the scores for steatosis, ballooned hepatocytes, and lobular inflammation, was calculated for all biopsies. P-values were determined by univariate ordinal logistic regression, to test for a change in histologic features with increasing grade of centrizonal arteries or microvessels. P-values were determined by the Cochran-Armitage test for trend for lobular inflammation and steatosis grade and by the chi-square test for ballooning (few vs. many), fibrosis (mild-moderate vs. advanced), and location of steatosis (Zone 3, azonal, or panacinar). The presence or absence of ductular reaction in the central zone was also noted as present or absent. Analyses were performed using SAS statistical software (version 9.1) and 2-sided p-values < 0.05 were considered statistically significant.
RESULTS
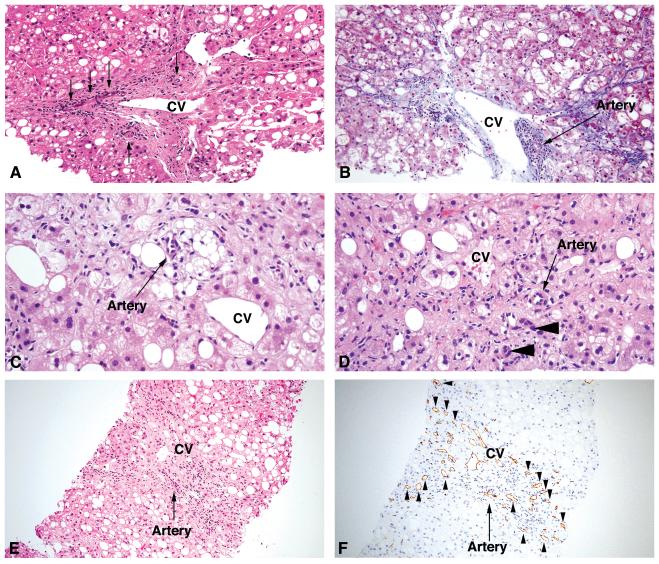
Arteries were identified in the centrizonal region in 40 of 100 cases (40%). In 18 instances, arteries were rare (grade 1) and in 22 they were common (grades 2-3) (Table 1). Arteries were identified in centrizonal areas more frequently in patients with higher stages of fibrosis (62%: 29 of 47 cases with stage 3 or 4 fibrosis) than in those with lower fibrosis stages (21%: 11 of 53 cases with stage 1b/1c or 2 fibrosis) (Table 1)(p < 0.001). Examples of arteries in centrizonal areas are shown in Figure 1 (A-D).
Figure 1.
(A) Central vein (CV) with surrounding scar and three small arteries indicated by arrows. Note absence of an interlobular bile duct and focal ballooned hepatocytes located just above the central vein, consistent with a centrizonal location. Fibrosis on this biopsy was Stage 3 (bridging fibrosis) (Hematoxylin-eosin (H&E) stain). (B) Central vein surrounded by scar (pericellular and sinusoidal pattern) with focal mononuclear inflammation (as well as a cluster of pigmented macrophages, left of the central vein) and an adjacent artery (arrow). Ballooned hepatocytes are also present in the zone above and to the right of the central vein. The fibrosis for this biopsy was Stage 1b (moderate centrizonal perisinusoidal fibrosis), confirming that centrizonal arteries can be present prior to the presence of portal-based fibrosis (Trichrome stain). (C) Lipogranuloma and artery in centrizonal region. Lipogranulomas are often located near the central vein, and the central vein in this case is noted to the right. Ballooned hepatocytes are present in this zone as well. The fibrosis for this biopsy was Stage 2 (centrizonal and periportal fibrosis only, with no bridging fibrosis identified) (H&E stain). (D) Scar in centrizonal region with ballooned hepatocytes, aberrant artery, and ductular reaction/metaplasia (arrowheads). Fibrosis on this biopsy was Stage 3 (bridging fibrosis) (H&E stain). (E) Central vein surrounded by scar with an adjacent artery (arrow). Fibrosis on this biopsy was stage 3 (bridging fibrosis) (H&E stain). (F) The same central vein surrounded by scar with microvessels (arrowheads indicate representative microvessels) and artery (arrow)(CD34 immunostain).
CD34+ microvessels were identified in the centrizonal region in 73 of 100 cases (73%). In 27 instances, microvessels were rare (grade 1) and in 46 they were common (grades 2-3) (Table 2). Microvessels were identified in centrizonal areas more frequently in patients with higher stages of fibrosis (87%: 41 of 47 cases with stage 3 or 4 fibrosis) than in those with lower fibrosis stages (60%: 32 of 53 cases with stage 1b/1c or 2 fibrosis)(p < 0.001). Examples of CD34+ microvessels in a centrizonal region are shown in Figure 1 (E-F).
Comparison of the presence of arteries to histologic features of NASH showed a significant association with stage of fibrosis, presence of ballooning degeneration, and with location of steatosis (Table 3). In contrast, higher centrizonal CD34+ microvessel prevalence (grades 2-3) showed a significant association with only fibrosis stage (Table 4).
Table 3. Comparison of histologic features of non-alcoholic steatohepatitis with presence of arteries.
| Histologic Feature | No arteries present (n = 60) |
Arteries present (n = 40) |
P-value* | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Ballooning score | 0.03 | |||||
| Few | 23 | 38.3 | 7 | 17.5 | ||
| Many | 37 | 61.7 | 33 | 82.5 | ||
|
Lobular
inflammation (20x magnification) |
0.95 | |||||
| < 2 foci | 19 | 31.7 | 16 | 40.0 | ||
| 2-4 foci | 32 | 53.3 | 15 | 37.5 | ||
| > 4 foci | 9 | 15.0 | 9 | 22.5 | ||
| Steatosis grade | 0.88 | |||||
| 5-33% | 20 | 33.3 | 17 | 42.5 | ||
| 34-66% | 26 | 43.3 | 11 | 27.5 | ||
| > 66% | 14 | 23.3 | 12 | 30.0 | ||
| Fibrosis stage | < 0.001 | |||||
| Mild- moderate (1b/1c-2) |
42 | 70.0 | 11 | 27.5 | ||
| Advanced (3-4) |
18 | 30.0 | 29 | 72.5 | ||
|
Location of
steatosis |
0.03 | |||||
| Zone 3 | 22 | 36.7 | 7 | 17.5 | ||
| Azonal | 13 | 21.7 | 18 | 45.0 | ||
| Panacinar | 25 | 41.7 | 15 | 37.5 | ||
|
NAFLD activity
score (NAS) |
0.60 | |||||
| 3-4 | 16 | 26.7 | 12 | 30.0 | ||
| 5-6 | 34 | 56.7 | 17 | 42.5 | ||
| 7-8 | 10 | 16.7 | 11 | 27.5 | ||
Cochran-Armitage Trend test used for lobular inflammation, steatosis grade and NAS; chi-square test used for ballooning, fibrosis and location of steatosis. NAFLD = Non-alcoholic fatty liver disease.
Table 4.
Comparison of histologic features of non-alcoholic steatohepatitis with microvessel grade.
| Histologic Feature | Grades 0 and 1 (n = 54) |
Grades 2 and 3 (n = 46) |
P-value* | |||
|---|---|---|---|---|---|---|
| N | % | N | % | |||
| Ballooning score | 0.10 | |||||
| Few | 20 | 37.0 | 10 | 21.7 | ||
| Many | 34 | 63.0 | 36 | 78.3 | ||
|
Lobular
inflammation (20x magnification) |
0.30 | |||||
| < 2 under | 19 | 35.2 | 16 | 34.8 | ||
| 2-4 under | 27 | 50.0 | 20 | 43.5 | ||
| > 4 under | 8 | 14.8 | 10 | 21.7 | ||
| Steatosis grade | 0.41 | |||||
| 5-33% | 18 | 33.3 | 19 | 41.3 | ||
| 34-66% | 23 | 42.6 | 14 | 30.4 | ||
| > 66% | 13 | 24.0 | 13 | 28.3 | ||
| Fibrosis stage | < 0.001 | |||||
| Mild- moderate (1b/1c-2) |
40 | 74.1 | 13 | 28.3 | ||
| Advanced (3-4) |
14 | 25.9 | 33 | 71.7 | ||
|
Location of
steatosis |
0.09 | |||||
| Zone 3 | 16 | 29.6 | 13 | 28.3 | ||
| Azonal | 12 | 22.2 | 19 | 41.3 | ||
| Panacinar | 26 | 48.2 | 14 | 30.4 | ||
|
NAFLD activity
score (NAS) |
0.47 | |||||
| 3-4 | 15 | 27.8 | 13 | 28.3 | ||
| 5-6 | 28 | 51.9 | 23 | 50.0 | ||
| 7-8 | 11 | 20.4 | 10 | 21.7 | ||
Cochran-Armitage Trend test used for lobular inflammation, steatosis grade and NAS; chi-square test used for ballooning, fibrosis and location of steatosis. NAFLD = Non-alcoholic fatty liver disease.
Ductular reaction was also common in centrizonal areas with fibrosis and arteries. Among the 40 biopsies with centrizonal arteries, 22 (55%) had ductular reaction. In addition, ductular reaction was more common with more advanced degrees of fibrosis, and was found in 4 of 11 cases (36%) with stage 1b/1c or 2 fibrosis compared to 18 of 29 cases (62%) with stage 3 or 4 fibrosis.
DISCUSSION
This study demonstrates that arteries and microvessels in scarred central zones are a common finding in patients with NASH and can be seen in this location as early as stage 1b (though interestingly not in the two cases of stage 1c included in this study). Isolated arteries in the hepatic lobule can be seen in liver with normal architecture and are generally believed to derive from hepatic arteries in portal zones (19). The finding of these centrizonal arteries in early stage disease also supports the idea that at least some of these arteries have appeared in or near the centrizonal region even before the development of bridging fibrosis from the portal area to the central zone. However, the increased prevalence of these arteries with increased stage of fibrosis suggests that they may branch and/or grow as part of remodeling of the vascular architecture in these scarred zones as fibrosis progresses in NASH. This process may be similar to what is known to occur as part of the formation of collateral vascular channels in the scarring of cirrhosis (20). Vascular reorganization may represent a compensatory or reparative mechanism of the liver in response to injury or disease-induced alterations in hepatocyte metabolism or hemodynamics (8, 16-18). Studies focusing on local stromal growth factors and chemokine variables elicited by the shift of hepatic stellate cells to a myofibroblast phenotype, and an associated change in matrix collagen content, suggest that molecular/cellular alterations could affect or promote growth of blood vessels in cirrhosis (25). In particular, microvessel formation, which is highlighted by CD34 immunolabeling in this case, is thought to represent evidence of angiogenesis (28) (27). Because hepatic stellate cells are the main fibrogenic cells in liver injury and are situated within the lobule, it would follow that they could significantly impact changes taking place in diseases where extracellular matrix deposition occurs in a perisinusoidal fashion, such as in NASH. Vasoactive substances such as endothelin-1 are found to be up-regulated in cirrhotic livers and may participate in the development of liver cirrhosis by also exerting an effect on hepatic stellate cells (11), which is in keeping with the idea that local factors may influence changes in the vascular network during development of hepatic fibrosis.
Although a causal link between obesity and insulin resistance with hepatic microvasculature perfusion deficits has been established (2), and various mediators have been implicated, precise cellular and molecular interactions remain to be fully determined. Attention has specifically been given to hepatic pro-angiogenic cytokines produced in response to hypoxic stimuli, particularly vascular endothelial growth factor (VEGF), and their effects on sinusoidal endothelial cells, hepatocytes, and hepatic stellate cells. These studies indicate that expression of VEGF (via regulation by hypoxia-inducible factor-1α, or HIF-1α), angiopoietin-1, and nitric oxide synthases are upregulated when these cells are subjected to low levels of oxygen (1, 6, 15). In response to worsening relative hypoxia in zone 3 as fibrosis progresses, stimulation of gene expression for VEGF and, thus, microvessel formation may increase, hence providing some basis for the theory that neovascularization of the central zone could be secondary to a hypoxia-related phenomenon.
This study also demonstrated that ductular reaction is a common finding in the centrizonal scar, and is also more prevalent in the later stages of fibrosis. Of note, ductular reaction in the periportal zone in patients with NASH has been associated with increased grade of NASH activity and portal inflammation, increased degree of hepatocytic replicative arrest, and is also correlated with progressive periportal fibrosis (22). These results were comparable to findings in chronic hepatitis C, where a similar association with periportal ductular reaction and fibrosis has been demonstrated (5). However, in the current cases, the centrizonal ductular reaction was obviously occurring some distance away from progenitor/stem cells typically thought to be the cell of origin of ductular reaction located in the periportal zone (13), (24). Thus, the unusual location may suggest that the change is a metaplastic response of hepatocytes to chronic injury and/or chronic ischemia, resulting in transformation to a more ductular phenotype rather than a progenitor cell reaction. Regardless of the cellular origin of the ductular reaction, these ductular structures could still possibly play an exacerbating role in the progression of fibrosis as well. The role and nature of these ductular structures could prove to be of interest in future studies.
The finding of ductular reaction in the centrizonal scar potentially compounds the risk of diagnostic error, as the presence of a centrizonal artery, particularly in samples with ductular reaction in the same scarred central zone, could potentially mimic the anatomy of a portal area, leading to the erroneous conclusion that the centrizonal region was a portal tract, and thus result in failure to diagnose NASH by a pathologist who may not be familiar with this finding. Also, even if NASH were correctly diagnosed, this finding could lead to overstaging (i.e. stage 1 to stage 2) or mis-staging (i.e. stage 1a/1b to 1c). The determination that the scar zone is not a remnant of a portal zone can be aided by careful histologic examination to determine whether the ductular structure and artery are embedded in the correct collagenous/elastic tissue background of a normal portal zone (26), as the normal portal zone has a more organized and uniform collagen and elastic fiber network than scar zones (10). In addition, the placements of the aberrant artery and a ductule in relationship to the adjacent hepatocytes are typically not in the correct anatomical configuration of a portal zone, in that the ductular structure or artery may lie too far apart with no visible portal vein candidate, or they may lie immediately adjacent to or among hepatocytes (instead of the two structures remaining separated from the “limiting plate” of the parenchyma by the types of collagen and elastic fibers seen in portal zones).
In summary, arteries and microvessels in the centrizonal region are a common finding in patients with NASH, especially in later stages of fibrosis. These arteries may arise in part through a process of microvessel formation related to local ischemia. The aberrant centrizonal artery needs to be recognized by pathologists as a feature of NASH so that the central zone is not mistaken for a portal zone and a correct diagnosis (and stage) of NASH can be rendered.
ACKNOWLEDGMENTS
The authors would like to thank Dr J Hoofnagle for his editorial input on this manuscript and Dr Nathan Bass in his role on the NASH CRN steering committee.
Supported in part by the Nonalcoholic Steatohepatitis Clinical Research Network (NASH CRN) (AS015 ancillary study, GRANT U01DK061738). The NASH CRN is a cooperative agreement supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) and the National Institute of Child Health and Human Development (NICHD) of the National Institutes of Health.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aleffi S, Petrai I, Bertolani C, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. doi: 10.1002/hep.20965. [DOI] [PubMed] [Google Scholar]
- 2.Brock RW, Dorman RB. Obesity, insulin resistance and hepatic perfusion. Microcirculation. 2007;14:339–347. doi: 10.1080/10739680701282986. [DOI] [PubMed] [Google Scholar]
- 3.Brunt EM, Janney CG, Di Bisceglie AM, et al. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark JM, Brancati FL, Diehl AM. The prevalence and etiology of elevated aminotransferase levels in the United States. Am J Gastroenterol. 2003;98:960–967. doi: 10.1111/j.1572-0241.2003.07486.x. [DOI] [PubMed] [Google Scholar]
- 5.Clouston AD, Powell EE, Walsh MJ, et al. Fibrosis correlates with a ductular reaction in hepatitis C: roles of impaired replication, progenitor cells and steatosis. Hepatology. 2005;41:809–818. doi: 10.1002/hep.20650. [DOI] [PubMed] [Google Scholar]
- 6.Corpechot C, Barbu V, Wendum D, et al. Hypoxia-induced VEGF and collagen I expressions are associated with angiogenesis and fibrogenesis in experimental cirrhosis. Hepatology. 2002;35:1010–1021. doi: 10.1053/jhep.2002.32524. [DOI] [PubMed] [Google Scholar]
- 7.Dunn W, Schwimmer JB. The obesity epidemic and nonalcoholic fatty liver disease in children. Curr Gastroenterol Rep. 2008;10:67–72. doi: 10.1007/s11894-008-0011-1. [DOI] [PubMed] [Google Scholar]
- 8.Elias H, Petty D. Terminal distribution of the hepatic artery. Anat Rec. 1953;116:9–17. doi: 10.1002/ar.1091160103. [DOI] [PubMed] [Google Scholar]
- 9.Farrell GC. Non-alcoholic steatohepatitis: what is it, and why is it important in the Asia-Pacific region? J Gastroenterol Hepatol. 2003;18:124–138. doi: 10.1046/j.1440-1746.2003.02989.x. [DOI] [PubMed] [Google Scholar]
- 10.Ferrell L, Belt P, N B. Arterialization of central zones in nonalcoholic steatohepatitis. Hepatology. 2007 Oct;46(Supple):732A. abstract 1113. [Google Scholar]
- 11.Ikura Y, Ohsawa M, Naruko T, et al. Expression of the hepatic endothelin system in human cirrhotic livers. J Pathol. 2004;204:304–310. doi: 10.1002/path.1644. [DOI] [PubMed] [Google Scholar]
- 12.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 13.Kuwahara R, Kofman AV, Landis CS, et al. The hepatic stem cell niche: identification by label-retaining cell assay. Hepatology. 2008;47:1994–2002. doi: 10.1002/hep.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lidofsky SD. Nonalcoholic fatty liver disease: diagnosis and relation to metabolic syndrome and approach to treatment. Curr Diab Rep. 2008;8:25–30. doi: 10.1007/s11892-008-0006-1. [DOI] [PubMed] [Google Scholar]
- 15.Medina J, Arroyo AG, Sanchez-Madrid F, et al. Angiogenesis in chronic inflammatory liver disease. Hepatology. 2004;39:1185–1195. doi: 10.1002/hep.20193. [DOI] [PubMed] [Google Scholar]
- 16.Mitra SK. The terminal distribution of the hepatic artery with special reference to arterio-portal anastomosis. J Anat. 1966;100:651–663. [PMC free article] [PubMed] [Google Scholar]
- 17.Ohnishi K, Chin N, Sugita S, et al. Quantitative aspects of portal-systemic and arteriovenous shunts within the liver in cirrhosis. Gastroenterology. 1987;93:129–134. doi: 10.1016/0016-5085(87)90324-6. [DOI] [PubMed] [Google Scholar]
- 18.Popper H. Pathologic aspects of cirrhosis. A review. Am J Pathol. 1977;87:228–264. [PMC free article] [PubMed] [Google Scholar]
- 19.Rappaport AM. The microcirculatory acinar concept of normal and pathological hepatic structure. Beitrage zu Pathologie. 1976;157:215–243. doi: 10.1016/s0005-8165(76)80083-2. [DOI] [PubMed] [Google Scholar]
- 20.Rappaport AM, MacPhee PJ, Fisher MM, et al. The scarring of the liver acini (Cirrhosis). Tridimensional and microcirculatory considerations. Virchows Arch A Pathol Anat Histopathol. 1983;402:107–137. doi: 10.1007/BF00695054. [DOI] [PubMed] [Google Scholar]
- 21.Rector RS, Thyfault JP, Wei Y, et al. Non-alcoholic fatty liver disease and the metabolic syndrome: an update. World J Gastroenterol. 2008;14:185–192. doi: 10.3748/wjg.14.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Richardson MM, Jonsson JR, Powell EE, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Schwimmer JB, Behling C, Newbury R, et al. Histopathology of pediatric nonalcoholic fatty liver disease. Hepatology. 2005;42:641–649. doi: 10.1002/hep.20842. [DOI] [PubMed] [Google Scholar]
- 24.Theise ND, Kuwahara R. The tissue biology of ductular reactions in human chronic liver disease. Gastroenterology. 2007;133:350–352. doi: 10.1053/j.gastro.2007.05.040. [DOI] [PubMed] [Google Scholar]
- 25.Tsutsumi M, Urashima S, Matsuda Y, et al. Changes in type IV collagen content in livers of patients with alcoholic liver disease. Hepatology. 1993;17:820–827. [PubMed] [Google Scholar]
- 26.Verrijken A, Francque S, Van Gaal L. The metabolic syndrome and the liver. Acta Gastroenterol Belg. 2008;71:48–59. [PubMed] [Google Scholar]
- 27.Weidner N. Measuring intratumoral microvessel density. Methods Enzymol. 2008;444:305–323. doi: 10.1016/S0076-6879(08)02814-0. Chapter 14. [DOI] [PubMed] [Google Scholar]
- 28.Weidner N, Semple JP, Welch WR, et al. Tumor angiogenesis and metastasis--correlation in invasive breast carcinoma. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]