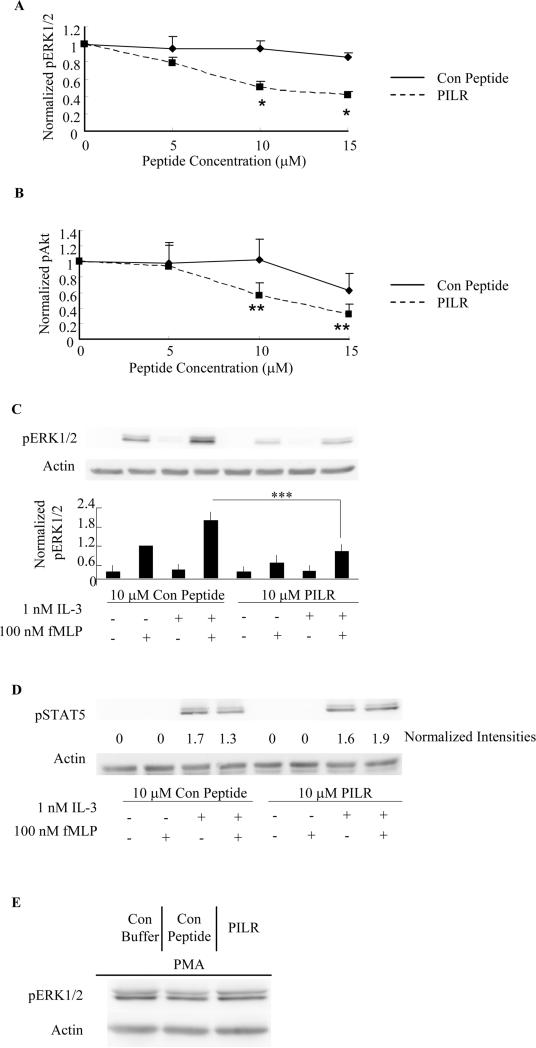
Figure 2. PILR inhibits fMLP-stimulated ERK1/2 and Akt phosphorylation in human blood eosinophils primed with IL-5 family members.
Blood eosinophils were preincubated with the Lyn recruitment antagonist PILR or control peptide for 30 min prior to priming with 100 pM IL-5 (A, B. D) or 1 nM IL-3(C) for 1 hr, followed by stimulation with 100 nM fMLP for 2 min. Phosphorylation of ERK1/2, Akt and STAT5 was assessed by SDS-PAGE and immunoblotting as detailed under Materials and Methods. Levels of actin were used as loading controls in all blots. Band intensities of pERK1/2 (A, *p<0.002, N=6, PILR vs. Con Peptide) and pAkt (B, **p<0.05, N=4, PILR vs. Con Peptide) were quantified by densitometry analysis and normalized to that of actin on the same blot. Data are summarized as the mean ± (SEM). C. Representative immunoblots are shown for five independent experiments. Data are summarized in the lower panel as the mean ± (SEM). ***p<0.05, N=5. D. Immunoblots of pSTAT5 and actin are shown as representatives of three independent experiments. Normalized band intensities expressed as fold induction are shown under the pSTAT5 immunoblot. E. Eosinophils were pretreated with control buffer, 10 μM control peptide or PILR for 30 min prior to the stimulation with 10 ng/ml PMA for 10 min. Representative immunoblots of pERK1/2 and actin of three independent experiments are shown.