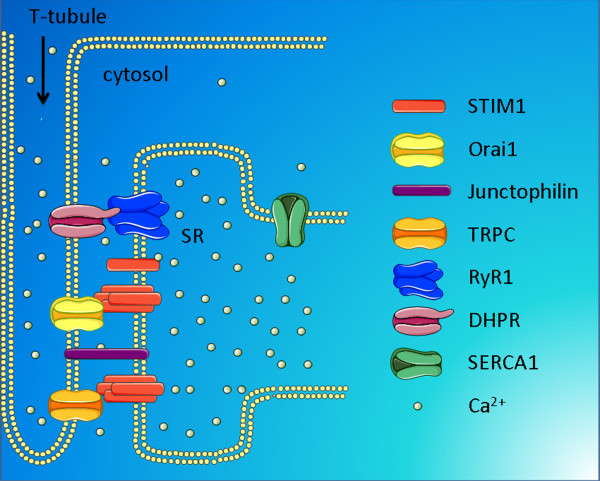
Figure 2.
Regulators of SOCE in skeletal muscle. At the triad junction, the voltage-sensitive DHPR (Cav1.1) and RyR physically interact. Depolarization of the plasma membrane causes activation of DHPR and subsequent opening of intracellular Ca2+-release channels, like the most abundant RyR isoform in skeletal muscle, RyR1. In addition, STIM1 monomers and/or oligomers accumulate at the terminal cisternae of the SR, thereby being in close proximity or in complex with Orai1. This pre-localization of STIM1 with Orai1 likely accounts for the fast-activation kinetics of SOCE. Furthermore, it may also account for a basal Ca2+ influx through Orai1 channels in resting conditions. As a consequence, STIM1/Orai1 complexes are an integral part of the Ca2+-homeostasis mechanisms responsible for maintaining proper SERCA1-mediated filling of the SR Ca2+ stores and sustaining resting [Ca2+] in the cytosol. The highly specialized and structural organization of the triad in close proximity of the SR to plasma-membrane SOCE, which involves junctophilin, seems to be essential for proper STIM1/Orai1-mediated Ca2+ homeostasis. A similar mechanism involving TRPC channels and STIM1 oligomers have also been implicated in Ca2+-influx mechanisms in the skeletal muscle, although STIM1-dependent regulation of TRPCs is a matter of debate. Abbreviations: DHPR, dihydropyridine receptor; RyR, ryanodine receptor; SOCE, store-operated Ca2+ entry; STIM, stromal interaction molecule; SR, sacroplasmic reticulum; TRPC, canonical transient receptor potential.