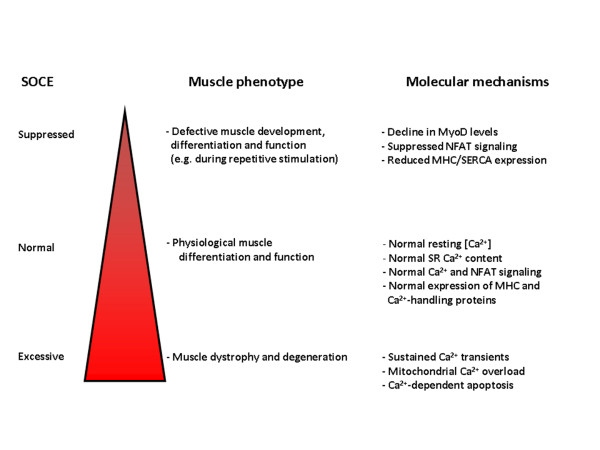
Figure 3.
Schematic overview of the physiological role of SOCE in skeletal muscle and pathophysiological consequences of Ca2+-influx dysregulation based on mouse-model studies. STIM1-controlled Ca2+ influx, either through Orai1 or TRPC channels, ought to be tightly regulated for proper muscle development and function. STIM1-gated Orai1-mediated Ca2+ influx seems to be a requisite for proper Ca2+ homeostasis in the skeletal muscle, maintaining resting cytosolic [Ca2+] sufficiently high and adequately filling the SR Ca2+ stores. This constitutes an essential mechanism that compensates for Ca2+ losses to the extracellular space. Importantly, these phenomena may not only act during periods of intense stimulation, but may also be required during basal conditions and regular muscle function. On the one hand, suppressing Ca2+ influx, for example in STIM1-deficient muscle fibers, leads to improper muscle development and function. This is likely due to hampered activation of NFAT-dependent signaling and defective expression of Ca2+-handling proteins, such as SERCAs and RyRs, as well as other proteins involved in muscle contraction. On the other hand, events such as mutations in dystrophin or overexpression of TRPC channels, lead to muscle dystrophy and degeneration, likely due to mitochondrial Ca2+ overload and Ca2+-dependent activation of the apoptotic program. Abbreviations: NFAT, nuclear factor of activated T cells; RyR, ryanodine receptor; SERCA, sarcoplasmic/endoplasmic-reticulum Ca2+-ATPase; SR, sacroplasmic reticulum; STIM, stromal interaction molecule; TRPC, canonical transient receptor potential.