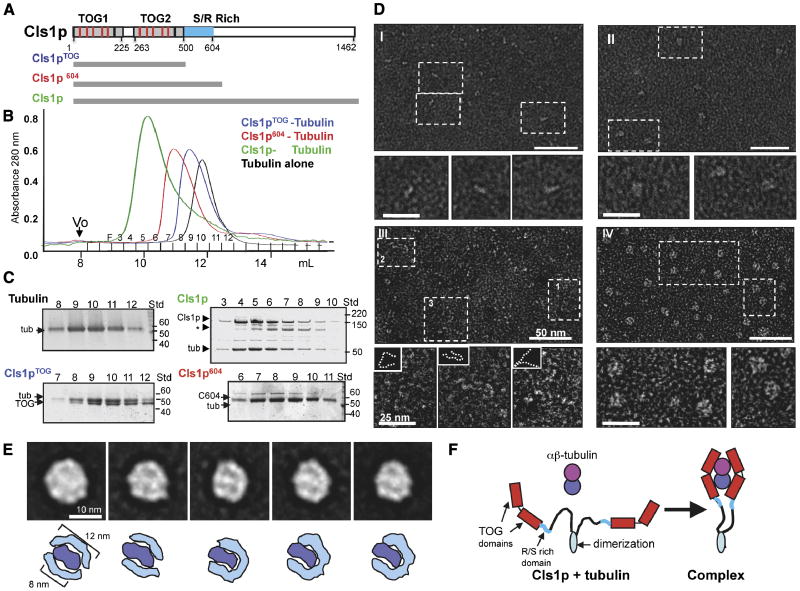
Figure 1. CLASP wraps around a tubulin dimer with two sets of TOG domains.
A) Domains of Cls1, as a typical CLASP protein. TOG-like and serine/arginine (S/R)-rich domains are in grey and blue, respectively. Studies with Cls1pTOG (residues 1-500), Cls1p604 (residues 1-604), and full length Cls1p (residues 1-1462) are described in this figure. CLASPs contain Two TOG-like domains each with five, tubulin-binding, intra-HEAT repeat turns (red lines in upper panel) identified by structure-based sequence alignments with XMAP215/Dis1 TOG domains (detailed in Fig S1A B).
B, C) Size exclusion chromatography of recombinant S. pombe Cls1p constructs. Intensity traces of Cls1pTOG (blue) and Cls1p604 (red) in complexes with soluble tubulin dimers indicate that Cls1p-tubulin complexes elute earlier than tubulin dimer alone (black). Cls1p-tubulin (green) elutes earlier than all other complexes, suggesting an extended conformation. SDS-PAGE of fractions from size exclusion chromatography (Panel C); Cls1pTOG and Cls1p604 co-elute with tubulin as complexes containing 1 Cls1p: 1 tubulin. The Cls1p604-tubulin complex elutes substantially earlier than Cls1pTOG-tubulin, indicating a higher Stokes radius (Supplementary Table S1). SDS-PAGE of fractions from Cls1p-tubulin complex peak shows that the peak saturates with a stoichiometry of 2 cls1p to 1 tubulin dimer, two-folds higher than the Cls1pTOG and Cls1p604 constructs. Asterisk (*) shows a very small amount of degraded inactive Cls1p. Supplementary data (Supplementary Table S1 Fig. S2) show that Cls1p is a dimer that binds a single tubulin dimer tightly, while Cls1pTOG and Cls1p604 are monomers that dissociate quickly from tubulin.
D) Negative stain electron microscopy of Cls1p and Cls1pTOG in the presence and absence of tubulin dimer. Panel I: Cls1pTOG; higher magnification images below panel I, show that the elongated Cls1pTOG molecules are ∼10 nm in length. In panel II, images of purified Cls1pTOG-tubulin dimer complexes immediately after size exclusion chromatography elution. Cls1pTOG appears to bind a tubulin dimer along its length. Panel III: Full-length Cls1p appears to be extended and contain flexible linkers. Panel IV: Full-length Cls1p-tubulin complexes are compact particles of uniform globular shape. Line traces of the lower magnification images in panel III are shown as broken lines as insets above each higher magnification image below panel III.
E) Upper panel: Images of five representative class averages from a reference-free classification of 2700 full length Cls1p-tubulin single particle images. The full image classification (Fig S2C) shows that Cls1p-tubulin complexes are homogenous with defined substructure. In each class average, two thin rim densities, about 12 nm in length encircle an elongated 8 nm density at the center of the particle. Lower panel: Interpretive drawing showing the outer rim densities as two sets of TOG domains (cyan) and the central density as a single tubulin dimer (blue).
F) Model for the CLASP conformational change accompanying the binding of a tubulin heterodimer. The C-terminal domains (900 residues) of Cls1p are flexibly linked with respect to the tubulin complex.