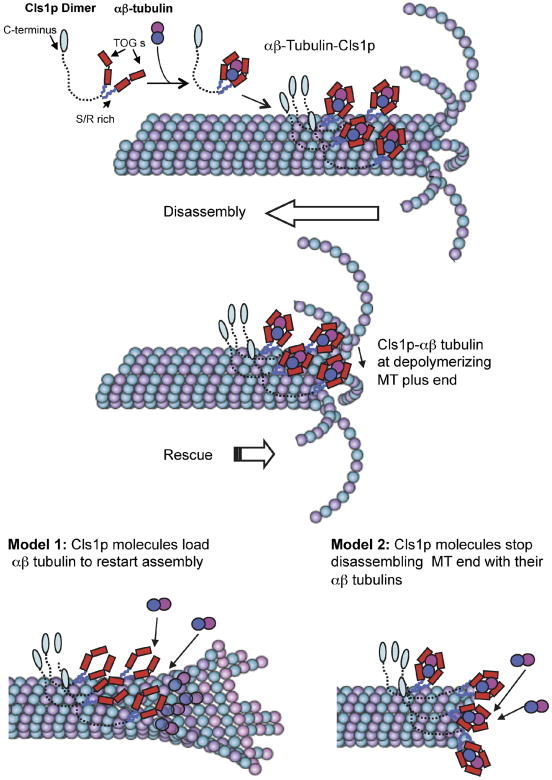
Figure 7. Mechanism of CLASP in promoting MT rescues.
The S. pombe CLASP, Cls1p, is a dimer that binds a single αβ-tubulin dimer through two sets of TOG domains and binds to the MT lattice through two S/R rich domains. The C-terminal domain, shown extended, may bind other molecules. Cls1p dimers loaded with αβ-tubulin dimer bind non-uniformly along the MT lattice. When the disassembling end reaches a position of high local Cls1p density, Cls1p promotes rescue by halting disassembly and stimulating re-growth. Two models may explain Cls1p activity: model 1 shows Cls1p molecules loading their bound tubulin to MT plus end to restart assembly (left). Model 2 shows Cls1p molecules using their bound tubulin to halt protofilament and pause MT plus end disassembly. Dynamic MT Images were adapted from Akhmanova and Steinmetz (2008).