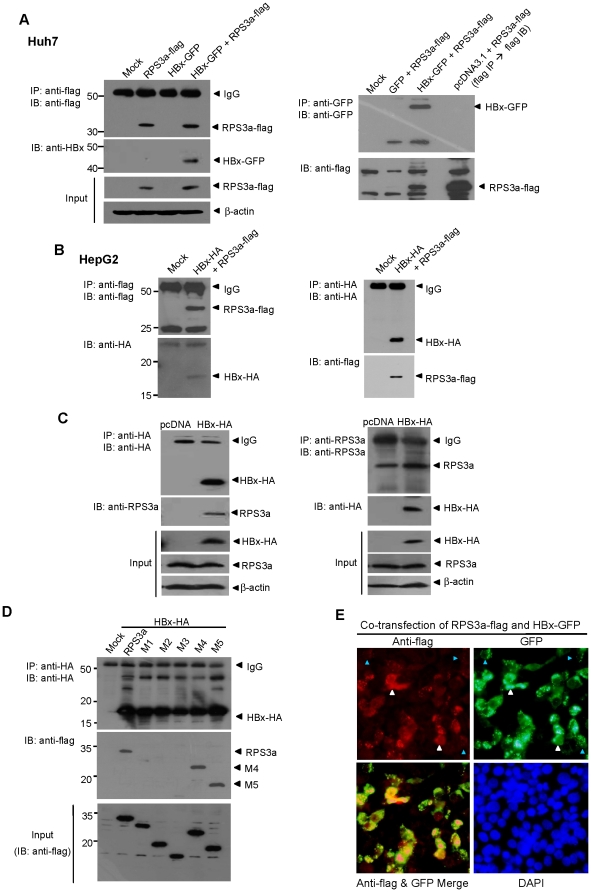
Figure 7. RPS3a interacts with HBx via its N-terminal domain and co-localizes in liver cells.
(A) RPS3a physically interacts with HBx in liver cells. pEG-HBx-GFP and pcD-RPS3a-flag were transfected in Huh7 cells. At 96 hr post-transfection, cell lysates were immunoprecipitated with anti-flag antibody (left) and anti-GFP antibody (right). Western blot analysis was performed using indicated antibodies. After stripping, each membrane was treated with anti-HBx antibody (left) and anti-flag antibody (right). As a positive control for identifying the RPS3a-flag band, immunoprecipitation was performed with anti-flag followed by western blot using the same antibody. (B) Interaction of HBx with RPS3a in HepG2 cells without GFP-fusion. The pcD-HBx-HA and pcD-RPS3a-flag were co-transfected in HepG2 cells and the immunoprecipitation and western blot were performed as indicated. (C) Interaction of HBx with endogenous RPS3a in Huh7 cells. The pcD-HBx-HA was transfected in Huh7 cells and immunoprecipitation and western blot were performed as indicated. (D) Identification of RPS3a binding domain critical for HBx interaction. Wild-type pcD-RPS3a-flag (2 µg) or pcD-mutants RPS3a-flag (M1∼M5) (2 µg) were co-transfected with pcD-HBx-HA (2 µg) in Huh7 cells. Aliquots of total lysates were used for western blot analysis (lower panel). The remaining lysates were immunoprecipitated with anti-HA antibody and detected by anti-flag antibody. After stripping, the membrane was reblotted by anti-HA antibody. (E) HBx and RPS3a co-localize in transfected liver cells. Huh7 cells were co-transfected with pEG-HBx-GFP (2 µg) and pcD-RPS3a-flag (2 µg). After 24 hr, immunocytochemistry analysis was performed using anti-flag antibody. White and blue triangles indicate the cells with high and low expression of RPS3a, respectively. Note that low expression of RPS3a is linked with punctate expression of HBx.