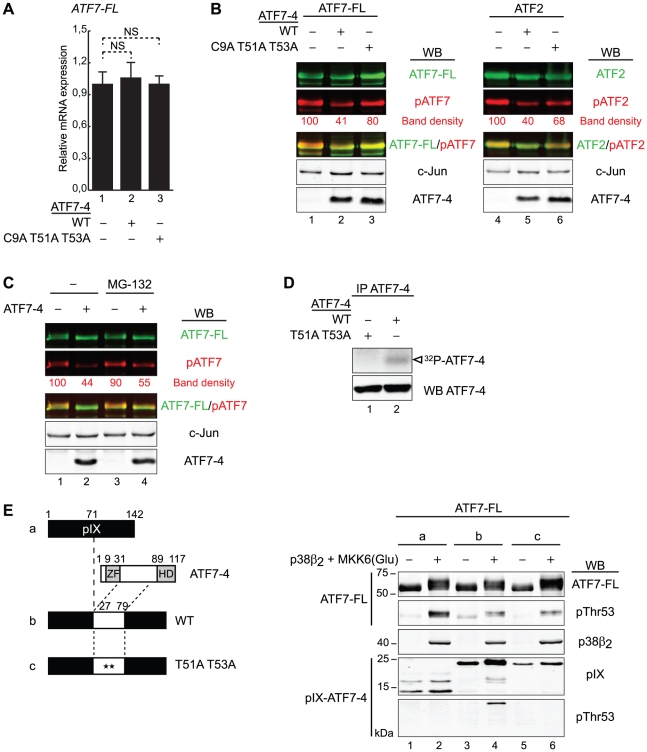
Figure 6. ATF7-4 impairs ATF7-FL/ATF2 phosphorylation by retaining a specific kinase.
(A) Endogenous ATF7-FL relative mRNA expression levels were assessed by real-time RT-PCR in HeLa cells, in presence of overexpressed ATF7-4 WT or mutant derivative. Data were normalized to hypoxanthine-guanine phosphoribosyltransferase (HPRT) gene expression. Results are the average of two independent experiments. Standard deviations and statistical significance by Student t-test are shown: NS = nonsignificant. (B and C) The phosphorylation state of overexpressed ATF7-FL and ATF2 was established in presence of ATF7-4 WT or mutant version, in HeLa cells treated (C) or not (B) with MG-132 proteasome inhibitor. The analysis was performed on total cell extracts by western-blotting (WB) with specific antibodies as indicated. Images of total ATF7-FL/ATF2 (green channel) and phosphorylated forms (red channel) were merged with LI-COR Odyssey software. c-Jun levels were used as a loading control. The relative band density of phosphorylated forms was quantified and normalized to c-Jun band density. Results of representative experiments are shown. (D) HeLa cells overexpressing ATF7-4 or mutant version were treated with MG-132. ATF7-4 and the associated proteins were immunoprecipitated with anti-ATF7-4 specific antibody, and assayed for a kinase assay with radiolabelled [γ-32P]ATP. The analysis was performed by autoradiography (top panel) and WB (bottom) in parallel. The white arrow indicates radiolabelled ATF7-4. (E) The phosphorylation state of overexpressed ATF7-FL was analyzed in presence of pIX (a) or pIX-ATF7-4 fusion proteins with the docking site of ATF7-4 for kinases, either WT (b) or mutant version (c). HeLa cells were co-expressed with p38β2 and MKK6(Glu). Cell lysates were analyzed by WB to detect the specific phosphorylation of ATF7-FL (two upper panels) and pIX-ATF7-4 fusion proteins (two lower panels).