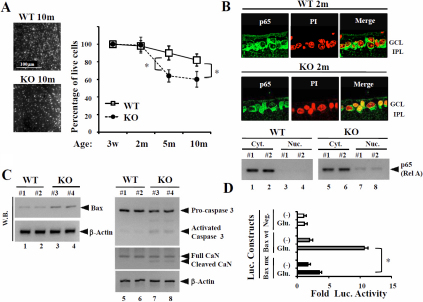
Figure 1.
Activation of pro-apoptosis factors, caspase 3, Bax, and p65(RelA), and calcineurin in the retina of p50-deficient mice. A: Age-related ganglion cell death in p50-deficient (KO) mice. Retrogradely labeled cells from 10 fields of identical size (230×150 μm) in flat-mounted retinas were counted under a fluorescence microscope. The fields were located at approximately the same distance from the orra serata (500 μm). Scale bar=100 μm. The average number of RGCs per field was calculated for each retina and analyzed by Bonferroni correction. Data are the mean±SEM (each 8~12; *p<0.01). B: Immunohistochemical staining for NF-κBp65 expression at 2 months in wild-type (WT) mice and p50-deficient mice. NF-κBp65 translocates into the nucleus of RGCs in KO mice. GCL=ganglion cell layer, IPL=inner plexiform layer. Green; p65, Red; PI. Original magnification; 120×. Western blot analysis of NF-κBp65 and Bax protein levels in KO mice and WT mice. Immunoreactive bands of NF-κBp65 in the nucleus were slightly more intense in KO than WT mice. C: western blot analysis of pro-apoptosis factors, Bax, caspase 3 and p65(RelA), and calcineurin (CaN) levels in KO mice and WTmice. Bax protein levels were also slightly increased in KO mice. Activated caspase 3 and cleaved CaN were detected in KO mice. D: The luciferase reporter vectors containing the wild-type or mutated B sites of the PMBaxSacI bax promoter (−386 to −1) or the corresponding empty luciferase reporter vector (shown in Appendix 1-Figure 1) were transiently co-transfected with or without the NF-κBp50 expression vector in RGC-5 cells. RGC-5 cells, pre-treated with tacrolimus, were stimulated by glutamate for the final24 h, and then luciferase activities were measured. Values were normalized to those obtained with the co-transfected pSV-β-galactosidase expression vector. Each assay was performed at least three times in triplicate. Luciferase-reporter assays showed that glutamate-treatment markedly induced Bax expression due to NF-κB activation. Data are presented as the mean for three independent experiments (*S.D.).