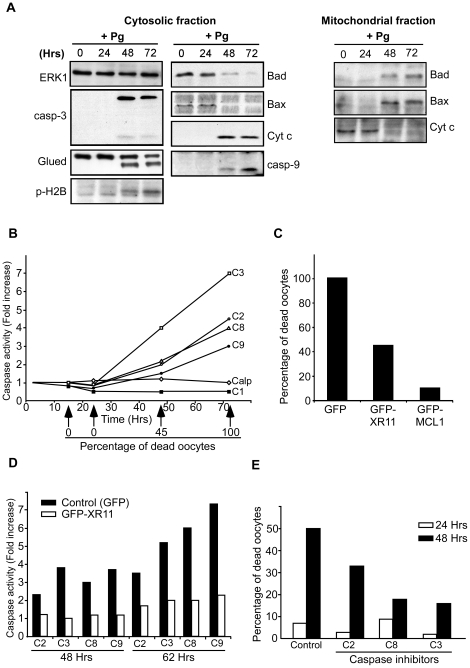
Figure 3. Mitochondrial- and caspase dependent apoptosis is responsible for unfertilized egg death.
Fully-grown prophase oocytes were isolated from the ovaries and stimulated by 1 µM progesterone. (A) Western blot analysis of cytosolic and mitochondrial fractions of unfertilized eggs was performed at various times following progesterone addition (+Pg) with antibodies against ERK1, the cleaved form of caspase 3 (casp-3), p150Glued, the Ser14-phosphorylated form of histone H2B (p-H2B), Bad, Bax, Cyt c and the cleaved form of caspase 9 (casp-9). (B) Caspase and calpain activities of unfertilized eggs were assayed at various times after progesterone stimulation with the following substrates: Suc-LY-AMC (calpain, Calp, white diamonds), Ac-YVAD-AMC (caspase 1, C1, dark squares), Z-VDVAD-AFC (caspase 2, C2, white circles), DEVD-AMC (caspase 3, C3, white squares), Z-IETD-AFC (caspase 8, C8, white triangles) and Ac-LEHD-AFC (caspase 9, C9, black circles). In parallel, the percentage of dead eggs was followed using morphological criteria. (C) Oocytes were microinjected with mRNAs encoding either GFP, GFP-XR11 or GFP-Mcl-1 fusion proteins and then incubated in the presence of 1 µM progesterone. The percentage of dead eggs was estimated 70 h later using morphological criteria. (D) Oocytes were microinjected with mRNAs encoding either GFP (control, black columns) or GFP-XR11 fusion protein (white columns). They were then incubated in the presence of 1 µM progesterone. Caspase 2 (C2), caspase 3 (C3), caspase 8 (C8) and caspase 9 (C9) activities were assayed 48 and 62 h later as described in (B). (E) Oocytes were microinjected with various caspase inhibitors: VDVAD-FMK (caspase 2, C2), DEVD-FMK (caspase 3, C3) or IETD-FMK (caspase 8, C8). Progesterone was added and the percentage of dead eggs was estimated 24 h (white columns) and 48 h (black columns) later after morphological criteria. All data shown in these panels are representative experiments repeated on oocytes and eggs from at least three different females.