Figure 2.
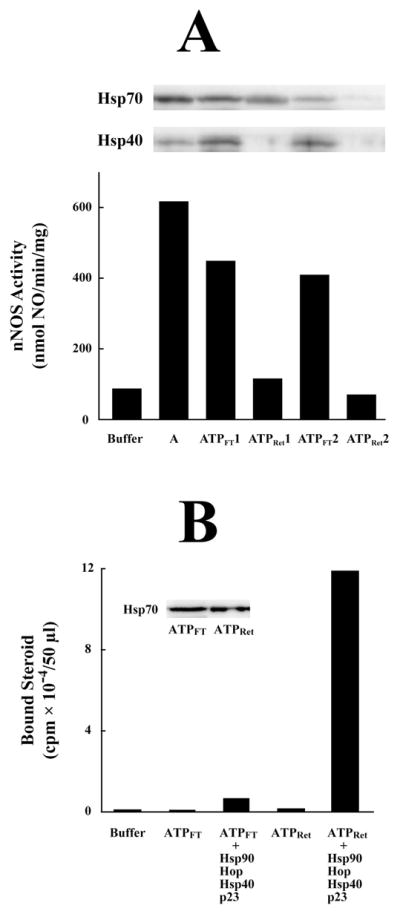
ATP-agarose fractionation of apo-nNOS activating activity in DE52 fraction A. (A) DE52 fraction pool A from reticulocyte lysate was applied to an ATP-agarose column, and the flow-through (ATPFT) and retained (ATPRet) fractions were prepared as described under Methods. The first ATP-agarose flow-through (ATPFT1) was applied to a fresh ATP-agarose column to prepare a second flow-through (ATPFT2) and retained (ATPRet2) preparation. The apo-nNOS activating activity of DE52 fraction A and the ATP-agarose flow-through and retained fractions was assayed. The Western blots show the Hsp70 and Hsp40 in aliquots of each preparation. (B) The Hsp70 in the ATPFT fraction does not activate the steroid binding activity of the GR in the presence of the other components of the chaperone machinery. Stripped GR was incubated with purified Hsp90, Hop, Hsp40 and p23 in the presence of ATPFT or ATPRet fractions as indicated. The amounts of ATPFT and ATPRet fractions were adjusted to contain similar amounts of Hsp/Hsc70 as indicated by the immunoblot in the inset.
