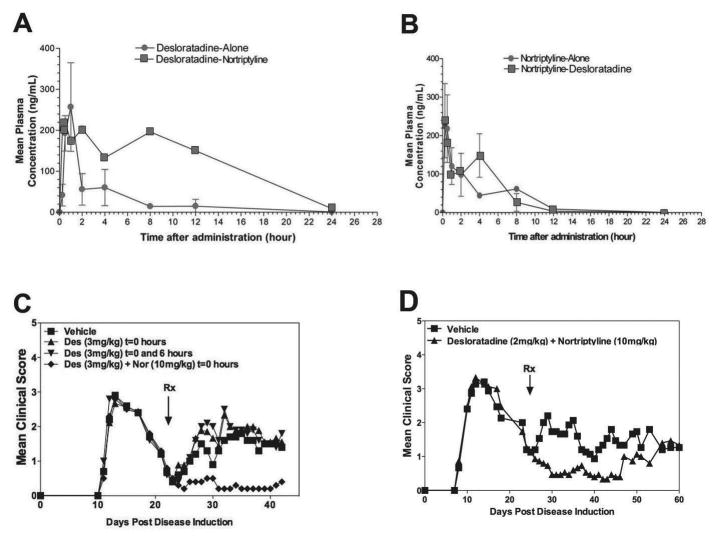
Fig 3. Increased efficacy of combination treatment with desloratadine and nortriptyline is not due to an increase in desloratadine half-life.
To determine the pharmacokinetics of CRx-153 in vivo female SJL/J mice (n=3–5 per PK time point) were dosed with 3 mg/kg desloratadine or 10 mg/kg nortriptyline alone (●), or in combination (■). Blood sampling for pharmacokinetics analysis was done after a single dose and after 21 days of chronic dosing. Samples were analyzed by a reversed phase HPLC/MS/MS method (A and B). Active R-EAE was induced in groups of 10 SJL/J mice with PLP139–151 in CFA on day 0. Following recovery from the acute disease episode (day +21), groups of 10 SJL/J mice were treated with either a Vehicle (■), desloratadine (3mg/kg) once daily (▲), desloratadine (3mg/kg) twice daily at 0 and 6 hours (▼), or a combination of desloratadine (3mg/kg) plus nortriptyline (10mg/kg) (◆) on twenty-one consecutive days via gavage, and monitored for clinical disease (C). PLP139–151 transfer disease was induced in SJL/J mice (10 mice per treatment group). At the onset of disease remission (day +23) mice were treated with Vehicle (■), desloratadine 2mg/kg plus nortriptyline (10mg/kg) (▲) on twenty-one consecutive days via gavage, and monitored for clinical disease (D). Clinical results are expressed as the mean clinical score. One representative experiment of two is shown.