Abstract
Aims
Catestatin (CST) is a chromogranin A (CgA)-derived peptide (hCgA352–372) with three identified human variants (G364S/P370L/R374Q-CST) that show differential potencies towards the inhibition of catecholamine release. Although CST affects several cardiovascular parameters, the mechanisms underlying CST action in the heart have remained elusive. Therefore, we sought to determine the mechanism of action of CST and its variants on ventricular myocardium and endothelial cells.
Methods and results
Contractile force and Ca2+ transients were measured, respectively, on rat papillary muscles and isolated cardiomyocytes (CC) under basal conditions and after β-adrenergic stimulation. Nitric oxide (NO) production and endothelial nitric oxide synthase (eNOS) phosphorylation (PSer1179eNOS) were studied in bovine aortic endothelial (BAE-1) cells. Under basal conditions, wild-type CST (WT-CST, 10–50 nM) transiently enhanced myocardial contractility. CST variants (G364S and P370L) exerted a comparable positive inotropic effect. The H1 histamine receptor antagonist mepyramine abolished the increase of contractile force induced by WT-CST. Moreover, WT-CST dose-dependently (5–50 nM) reduced the effect of β-adrenergic stimulation. This anti-adrenergic effect was not mediated by a direct action on CC, but involved a PI3K-dependent NO release from endocardial endothelial cells. Indeed, CST induced a wortmannin-sensitive, Ca2+-independent increase in NO production and eNOS phosphorylation on BAE-1 cells. While the anti-adrenergic and NO release effects of P370L-CST were comparable with those of WT-CST, the G364S variant was ineffective on the same parameters.
Conclusion
Our results suggest that the anti-adrenergic action of CST depends on the endothelial PI3K–Akt–eNOS pathway and that its structural alterations entail functional features that correlate with the different anti-hypertensive potential described in humans.
Keywords: Myocardial contractility, Nitric oxide, Endothelial cells, Cardiomyocytes, β-adrenergic stimulation
1. Introduction
Chromogranin A (CgA) is a 48 kDa acidic secretory protein, highly conserved in the vertebrate secretory granules of both the diffuse neuroendocrine system1,2 and in the heart,3 where it is co-stored and co-secreted with catecholamines and natriuretic peptides. CgA was initially used as a biomarker of neuroendocrine tumours,4 but it has recently emerged as a marker of cardiovascular dysfunctions, such as essential hypertension,5–7 hypertrophic/dilatative cardiomyopathy, and heart failure.3,8 Circulating levels of CgA are increased in patients after myocardial infarction9 and provide prognostic information in acute coronary syndromes.7
The involvement of CgA in cardiovascular function is attributed to its function as a prohormone. Via post-translational proteolytic processing, it gives rise to bioactive peptides implicated in various counter-regulatory processes, such as vasostatins and catestatin (CST).1,10 Although CST was initially identified as a potent endogenous nicotinic–cholinergic antagonist, subsequent studies indicated that CST exerts signalling effects in several organs/systems, including cardiovascular system. CST has recently been shown to act as a novel regulator of cardiac function and blood pressure.10,11 CST induces vasodilation by both antagonizing nicotine-evoked catecholamine secretion from chromaffin cells,4,5,11–16 and causing the local release of histamine.17 In humans, CST administration caused vasodilation,18 and CST plasma levels are decreased not only in hypertensive patients but also in their still-normotensive offsprings.12 Consistent with human studies, it has been shown that exogenous CST rescues arterial hypertension5 and improves dampened baroreflex sensitivity19 and heart rate variability20 in CgA knockout mice. In vitro studies on the isolated rat heart showed that CST elicits negative inotropic and lusitropic actions, as well as a vasorelaxant influence on coronary arteries pre-contracted by endothelin-1, through a Gi/o protein–nitric oxide (NO)–cGMP-dependent mechanism.10 Moreover, CST exerts cardioprotective effects against contractile dysfunction and cell death induced by ischaemia and reperfusion in isolated rat heart and ventricular cells.21 Three naturally occurring amino acid substitution variants of CST have been described in humans.22 Although two of the CST variants (Pro370Leu and Arg374Gln) are relatively rare (minor allele frequencies 0.3–0.6%, respectively), the Gly364Ser variant showed an allele frequency of about 3–4%. These variants displayed differential potencies toward inhibition of nicotinic cholinergic agonist-evoked catecholamine secretion from chromaffin cells in vitro [Pro370Leu > wild-type (WT) > Gly364Ser > Arg374Gln].15 Of note, human carriers of the Gly364Ser allele showed marked modification in both the parasympathetic and sympathetic activity, and may present a minor risk to develop hypertension, especially evident in males.6 These findings prompted us to test the effects of WT-CST and its variants on the heart. Moreover, since the molecular mechanisms underlying the cardioinhibitory and vasorelaxing effects of CST remain to be fully elucidated, we investigated the signalling pathways activated by CST in mammalian cardiac and endothelial cells that may lead to modulation of cardiac contractility and coronary tone.
2. Methods
2.1. Animal care and sacrifice
Experiments were performed on rats which were allowed ad libitum access to tap water and standard rodent diet. The animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996), and in accordance with Italian law (DL-116, 27 January 1992). The scientific project was supervised and approved by the Italian Ministry of Health, Rome, and by the ethical committee of the University of Torino. Young adult rats were anaesthetized by i.p. injection of pentobarbital (nembutal, 100 mg/kg) and killed by stunning and cervical dislocation.
2.2. Solutions and drugs
Ca2+-free Tyrode solution and Tyrode standard solution compositions are provided in the Supplementary material. All drug-containing solutions were prepared fresh before the experiments. WT-CST and its variants (G360S-CST and P370L-CST) used were synthesized by the solid-phase method, using 9-fluorenylmethoxy-carbonyl protection chemistry.14 If not otherwise indicated, chemicals were purchased from Sigma.
2.3. Isolated papillary muscle
Papillary muscles dissection and mounting were performed as previously described.23 Briefly, papillary muscles were dissected free from the left ventricle under a stereomicroscope and superfused with oxygenated Tyrode solution at 37°C. Papillary muscles were driven at constant frequency (120 bpm) with a pair of electrodes connected to a stimulator (302 Anapulse, W. P. Instruments, New Haven, CT, USA) via a stimulus isolator (model 305-R, W. P. Instruments) operating in the constant-current mode. Isometric twitches were evaluated by a transducer (model 60-2997, Harvard Instruments) and continuously acquired and recorded by a PowerMac computer using Labview software (National Instruments).
Before each experiment, papillary muscles were equilibrated in oxygenated (100% O2) Tyrode solution for 30 min. The effects of different concentrations of WT-CST (2–50 nM) and the two naturally occurring variants, G364S-CST and P370L-CST (in both cases, 5–50 nM), were studied both under basal experimental conditions and in the presence of β-adrenergic stimulation (Isoproterenol, Iso, 50 nM). Each treatment lasted 10 min. All solutions containing drugs were prepared immediately before the experiments. In some experiments, papillary muscles were treated with 0.5% Triton X-100 for 1–2 to remove endocardial endothelium (EE). Mepyramine (0.5 μM), NG-nitro-L-arginine methyl ester (L-NNA, 1 mM), wortmannin (100 nM), or 1H-[1,2,4]oxadiazole-[4,4-a] quinoxalin-1-one (ODQ, 10 mM) were used to block H1 receptor, NO synthesis, PI3K, or guanylate cyclase activity, respectively.
2.4. Isolated rat ventricular cells
Isolation of rat ventricular cardiomyocytes and calcium transient measurements were performed as previously described.23 After sacrifice, the rat hearts were explanted, washed in modified Ca2+-free Tyrode solution, and cannulated via the aorta. All the following operations were carried on under a laminar flow hood. The heart was perfused at a constant flow rate of 10 mL/min with Ca2+-free Tyrode solution with a peristaltic pump for approximately 5 min (37°C) to wash away the blood and then with 10 mL of Ca2+-free Tyrode supplemented with collagenase (0.3 mg/mL) and protease (0.02 mg/mL). Hearts were then perfused and enzymatically dissociated with 30 mL of Ca2+-free Tyrode containing 50 µM CaCl2 and the same enzymatic concentration mentioned before. Atria and ventricles were then separated and the ventricles were cut in small pieces and shaken for 10 min in 20 mL of Ca2+-free Tyrode solution in the presence of 50 µM CaCl2, collagenase, and protease.
2.5. Measurement of calcium transients
Methods for calcium transient measurements on isolated rat ventricular cells are provided in the Supplementary material.
2.6. Bovine aortic endothelial-1 cells, Ca2+/NO fluorimetric measurements
The effect induced by WT-CST on intracellular Ca2+ concentration and NO production was studied in bovine aortic endothelial (BAE-1) cells by confocal microscopy. Methods for BAE-1 culture and for simultaneous Ca2+/NO fluorimetric measurements were previously described.23 Additional Materials and Methods are provided in the Supplementary material.
2.7. BAE-1 cells, immunofluorescence, and western blot
Immunofluorescence for endothelial nitric oxide synthase (eNOS)/PeNOSSer1179 and western blot experiments for eNOS/PeNOSSer1179 and Akt/PAktSer473 detection on BAE-1 cells were performed as described previously,24 with a monoclonal anti-eNOS antibody (BD Biosciences, 1:50 for immunofluorescence, 1:200 for western blot), a polyclonal anti-eNOSSer1179 antibody (Invitrogen, 1:10 for immunofluorescence, 1:100 for western blot), a monoclonal anti-Akt (Cell Signaling, 1:1000) and a polyclonal anti-PAktSer473 (Cell Signaling, 1:1000).
Additional Materials and Methods are provided in the Supplementary material.
2.8. Statistical analysis
Values are shown as means ± SEM. Statistical analysis was performed by ANOVA followed by the Newman–Keuls multicomparison test. A P-value < 0.05 was considered significant.
3. Results
3.1. Isolated papillary muscle
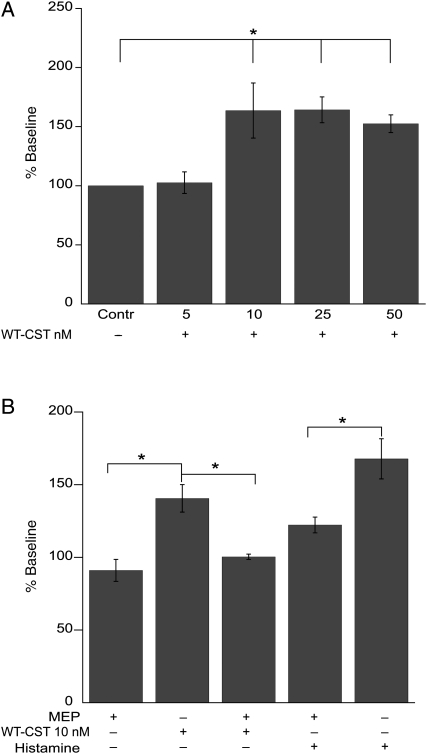
We observed that, under basal conditions, a low concentration (5 nM) of WT-CST had no significant effect on myocardial contractility, whereas higher concentrations (10–50 nM) induced a transient positive inotropic effect (about 50% over control; Figure 1A), reaching a peak after 2–3 min of treatment, which was completely reverted in 5–6 min when treated with 10 nM WT-CST. Such inotropic effect partially persisted at the end of treatment (10 min) at higher doses of CST (112.2 ± 9.4 and 107.9 ± 4.9% of control; 25 and 50 nM, respectively). The positive inotropic effect was completely abolished when papillary muscles were pretreated with the H1 receptor antagonist mepyramine (0.5 μM, 100.4 ± 1.9 vs. 140.6 ± 9.5%, n = 5; P = 0.01). At this concentration, mepyramine reduced (by 27%) the increased contractile force induced by 1 μM histamine (Figure 1B).
Figure 1.
Role of H1 histamine receptor activation in the early positive inotropic effect induced by WT-CST in isolated rat papillary muscle. (A) While 5 nM WT-CST was ineffective, higher concentrations (10–50 nM) transiently enhanced contractile force. (B) The H1 histamine receptor antagonist mepyramine (MEP; 0.5 μM) blocked the positive effect induced by WT-CST (10 nM) or histamine (1 μM). Data expressed as mean ± SEM% of baseline values from five experiments for each group (*P < 0.05). Baseline peak tension value was 258.8 ± 16.7 mg.
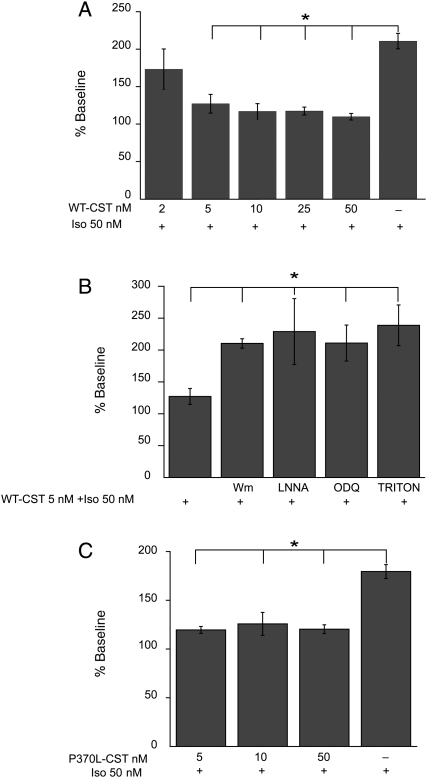
While 2 nM WT-CST did not significantly modify the response of papillary muscles to β-adrenergic stimulation (50 nM Iso) (184.8 ± 22.5 vs. 195.0 ± 19.9% of control), higher concentrations (5–50 nM) exerted significant anti-adrenergic effect (Figure 2A). Although 5 nM WT-CST was ineffective under basal conditions, it was able to significantly reduce the effect of Iso. The anti-adrenergic effect of 5 nM WT-CST (127.2 ± 12.5 vs. 214.5 ± 12.8%, Iso alone; n = 5 and 14, respectively) was abrogated when papillary muscles were pretreated with the chemical inhibitors of PI3-K (100 nM wortmannin), NO synthase (1 M L-NNA), or cGMP (10 μM ODQ) (Figure 2B), thus suggesting that PI3-K, NO, and cGMP play an important role in the inotropic effects induced by WT-CST.
Figure 2.
Anti-adrenergic effect of WT-CST and P370L-CST. (A) WT-CST reduced the response to Iso (50 nM) in a dose-dependent manner (5–50 nM). (B) The anti-adrenergic effect of WT-CST depends on endothelial cells and involves the PI3K–NO–cGMP pathway. Pretreatment of papillary muscles with 100 nM wortmannin, 1 m ML-NNA, 10 mM ODQ, or 0.5% Triton X-100 significantly reduced the effect of 5 nM WT-CST. (C) The P370L-CST variant exerted an anti-adrenergic effect comparable to that induced by WT-CST. Data expressed as mean ± SEM% of baseline values from five experiments for each group (*P < 0.05).
Finally, to test the involvement of NO released from endocardial endothelial cells, papillary muscles were treated with WT-CST after removal of endothelium by 0.5% Triton X-100. This protocol did not modify the inotropic effect induced by Iso under basal conditions (225.0 ± 10.0% of control, n = 5), whereas the anti-adrenergic effect of WT-CST was completely abolished (Figure 2B: 232.8 ± 32.0% of control, n = 5).
None of the pharmacological inhibitors used altered per se the positive inotropic effect induced by 50 nM Iso (212.6 ± 42.8, 227.2 ± 37.5, and 196.4 ± 44–2% of baseline values, in the presence of wortmannin, L-NNA, or ODQ, respectively). Wortmannin, L-NNA, and ODQ enhanced developed tension per se (147.6 ± 18.2, 119.8 ± 7.7, and 110.3 ± 2.0% of developed force recorded at the end of equilibration, respectively). Triton X-100 reduced contractile force to 60.5 ± 8.5% of baseline.
We performed additional experiments to study the effects of two variants of CST (G364S-CST and P370L-CST). Our results show that, under basal conditions, the effects of both G364S-CST and P370L-CST were comparable to that caused by WT-CST, being ineffective at a low concentration (5 nM), while higher concentrations (10–50 nM) induced a transient positive inotropic effect (126.3 ± 3.0 and 126.8 ± 4.0; 144.0 ± 23.2 and 123.2 ± 4.9; G364S-CST and P370L-CST 10 and 50 nM, respectively; all data expressed as percent of control from five experiments). Only P370L-CST was able to reduce the positive inotropic effect exerted by β-adrenergic stimulation with Iso (P < 0.05, Figure 2C). In contrast, G364S-CST failed to modulate the effect of Iso (Iso alone: 206.0 ± 28.1% of control; 188.7 ± 22.6 and 198.8 ± 41.5% of control, Iso in the presence of G364S-CST 10 and 50 nM, respectively; P = ns).
3.2. Ca2+ transients measurements on isolated ventricular cells
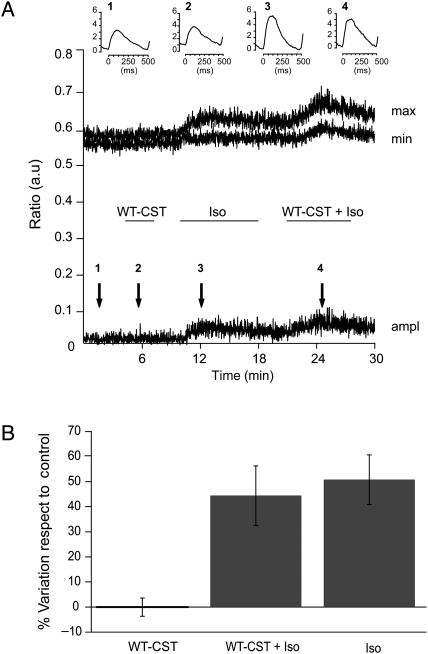
To discriminate between the direct inotropic effect of WT-CST and the indirect effect through release of NO from EE, we performed Ca2+ transient measurements on isolated cardiomyocytes. Here we used 5 nM WT-CST, the minimal concentration able to reduce the enhancement of contractile force induced by Iso without altering the basal contractility in papillary muscle (see previous section). We found (Figure 3) that WT-CST had no effect on the amplitude of Ca2+ transients recorded both under basal conditions or in the presence of Iso stimulation (% variation respect to control: 0.01 ± 3.7% WT-CST, n = 11; 44.4 ± 11.9% Iso + WT-CST, n = 6; 50.8 ± 9.9% Iso alone, n = 11).
Figure 3.
WT-CST is ineffective on Ca2+ transients in electric-field stimulated cardiomyocytes. (A) Time course of the maximum (max) and minimum (min) value and amplitude (ampl) of Ca2+ transients from a representative experiment, showing the lack of effect of WT-CST in both basal and Iso-stimulated conditions. The horizontal bars correspond to the times of drugs application. Inserts represent the mean of 20 transients corresponding in the time course to the different conditions (1 = control, 2 = WT-CST, 3 = Isoproterenol, 4 = WT-CST and Isoproterenol). (B) Bar graph representing the % variation respect to control of the amplitude of Ca2+ transients in the presence of 5 nM WT-CST, 5 nM WT-CST + µM Iso and Iso alone.
3.3. Nitric oxide production by BAE-1 cells
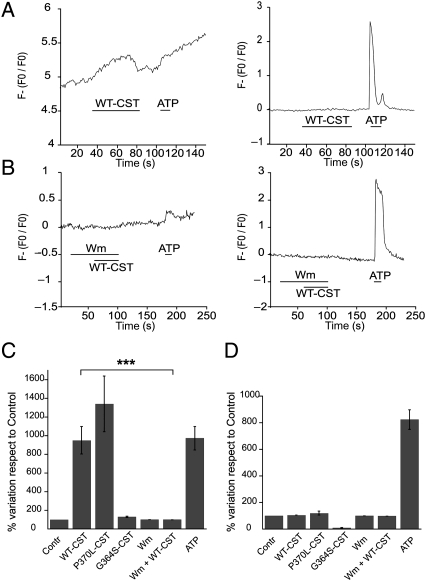
Taken together, the experiments performed on papillary muscles and on isolated ventricular myocytes suggest that the anti-adrenergic effect of both WT-CST and P370L-CST depends mainly on NO release from endothelial cells. To test whether these variants were able to activate eNOS in endothelial cells, we performed simultaneous measurements of NO and intracellular Ca2+ with specific fluorescent probes (Fluo-3AM and DAR-4AM) on BAE-1 cells. As a positive control, 100 µM ATP was used to induce Ca2+-dependent NO production. Consistent with the papillary muscle experiments, we observed that WT-CST and P370L-CST (5 nM) enhanced NO production, while G364S-CST was ineffective on NO synthesis (Figure 4A, left graph, and C). The percent variations respect to control of the first derivatives of the fluorescence signal (Figure 4C) were, respectively, 949.0 ± 147.2 for WT-CST (n = 41), 1399.5 ± 380.5 for P370L-CST (n = 16), 130.4 ± 7.2 for G364S-CST (n = 24), and 937.3 ± 125.5 % for ATP (n = 41).
Figure 4.
Augmented NO production in BAE-1 cells by WT-CST and P370L-CST. (A) Normalized fluorescence of DAR-4M AM (left traces) and Fluo3 AM (right traces) on single BAE-1 cells. WT-CST enhanced NO production without affecting intracellular calcium concentration. (B) In BAE-1 cells pretreated with Wm, WT-CST failed to induce NO synthesis stimulation. Bars in the time courses indicate the times of drugs application. (C) Bar graphs representing the percent variation respect to control of the first derivative values of the DAR-4M AM signal (to evidentiate changes in NO synthesis, left panel) and the percent variation respect to control of the normalized Fluo3 AM signal (right panel), in the different experimental conditions.
Interestingly, in contrast to ATP, the enhancement of NO production induced by WT-CST as well as P370L-CST was not accompanied by any variation in intracellular Ca2+ concentration (Figure 4A, right graph, and D; % variation respect to control: 104.5 ± 0.8 for WT-CST, n = 42; 119.2 ± 14.6 for P370L-CST, n = 18; 90.9 ± 2.6 for G364S-CST, n = 24; 1310.1 ± 29.6 for ATP, n = 42).
In addition, to investigate the involvement of PI3K in the NO increase caused by WT-CST, we performed simultaneous NO and Ca2+ measurements in BAE-1 cells pretreated with wortmannin (100 nM). While PI3-K blockade did not alter the basal NO production, it completely abrogated the enhancement of NO synthesis induced by WT-CST (Figure 4B and C). The percent variations respect to control of the first derivatives of the fluorescence signal were, respectively, 100.6 ± 0.9% for Wm and 100.5 ± 1.0% for Wm + WT-CST (n = 29).
3.4. Western blot and immunofluorescence experiments
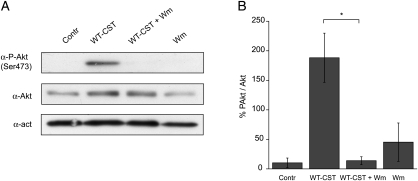
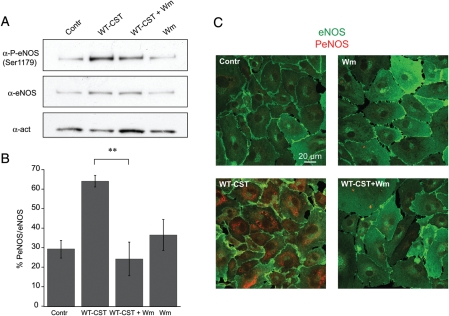
To gain better insights into the mechanisms involved in eNOS activation induced by WT-CST in BAE-1 cells, we performed western blot experiments on the phosphorylation status of Akt and eNOS. We found that 5 nM WT-CST induced a significant increase in both Akt and eNOS phosphorylation, an effect which was completely blocked by pretreatment with 100 nM wortmannin [%PAkt/Akt were, respectively, 10.3 ± 8.1 in control condition, 188.2 ± 41.7 after 15 min 5 nM WT-CST, 45.3 ± 32.5 after wortmannin alone, and 13.9 ± 6.7 after 5 nM WT-CST + wortmannin; n = 3 (Figure 5A and B); % PeNOS/eNOS were, respectively, 29.4 ± 4.5 in control condition, 64.1 ± 3.0 after 15 min 5 nM WT-CST, 36.5 ± 7.9 after wortmannin alone, and 24.2 ± 8.6 after 5 nM WT-CST + wortmannin; n = 3 (Figure 6A and B)]. The eNOS activation induced by WT-CST was further confirmed by immunofluorescence experiments (Figure 6C).
Figure 5.
Induction of a PI3-K-dependent Akt phosphorylation by WT-CST (5 nM) in BAE-1 cells. (A) Representative western blot experiment showing that WT-CST induced a wortmannin-sensitive increase in the level of PSer473Akt. (B) PSer473Akt/Akt ratio of densitometric values from western blots. Data are given as % values ± SE. *P < 0.05.
Figure 6.
Induction of a PI3-K-dependent eNOS phosphorylation by WT-CST (5 nM) in BAE-1 cells. (A) Representative western blot experiment showing that WT-CST induced a wortmannin-sensitive increase in the level of PSer1179eNOS. (B) PSer1179eNOS/eNOS ratio of densitometric values from western blots. Data are given as % values ± SE. **P < 0.01. (C) Confocal immunofluorescence imaging on BAE-1 cells showing that eNOS phosphorylation (PSer1179, red signal) is increased in WT-CST treated BAE-1 cells and that this effect is reverted by wortmannin pretreatment. Blots and immunofluorescence images are representative of a minimum of three experiments.
4. Discussion
The present study provides novel information on the cellular mechanisms underlying the cardiac antiadrenergic action of CST, highlighting the crucial involvement of a Ca2+-independent/PI3-K-dependent NO release from endothelial cells. In the last several years, CST has progressively been emerged as a peptide with important cardiovascular actions. Like other CgA-derived peptides, such as VS-1, CST at nanomolar concentrations induces negative inotropic effects on isolated heart preparations from different animal species, both under basal conditions and after β-adrenergic stimulation.10,25 Therefore, it has been suggested that CST, in addition to its relevant vasoreactivity observed in animal models11 and in man,12,18,26 acts as a cardioregulatory mediator, being able to exert an antiadrenergic action that could protect the heart against intense excitatory stimulations, such as those elicited by the stress response.2,27–29
Our experiments on isolated papillary muscle show that 10–50 nM CST exerts a biphasic effect on cardiac inotropism, i.e. an early, transient increase in contractile force, followed by a negative, antiadrenergic effect, comparable with that previously observed in the isolated heart.10,24 Previous reports indicate that 0.01–5 μM CST acts as a potent in vitro activator of histamine release from rat mast cells,30 and that this release, through H1 receptors stimulation, mediates, at least in part, the in vivo vasodilator effect of CST.17 Moreover, histamine exerts a positive inotropic effect on rat ventricular myocardium,31 probably acting on H1 histamine receptors, which are abundantly expressed in this tissue.32
In the light of these prerequisites, the complete blockade of WT-CST (10 nM)-induced early transient increase in contractile force by the H1 histamine receptor antagonist mepyramine strongly suggests that this effect is due to histamine release possibly from mast cells present within cardiac tissue and cardiac H1 receptors activation.
On the other hand, we found that 5 nM WT-CST was ineffective on basal papillary muscle contractility, while it was able to significantly reduce the effect of Iso, thus representing the minimal concentration able to reduce the enhancement of contractile force induced by Iso without altering the basal contractility of papillary muscle through H1 receptors activation.
It should be pointed out that the antiadrenergic effect that we report here for CST is comparable to the circulating concentrations of CgA found in healthy humans (0.5–2 nM),33 or in patients with severe heart failure (5–20 nM).8 In addition, CgA has also been detected in different myocardial cells, such as in the secretory granules of rat atrial myoendocrine cells, colocalized with atrial natriuretic peptides,34 as well as in rat Purkinje cells, and H9c2 cardiomyocytes.35 Moreover, Biswas et al.36 have recently shown that CST-containing peptides are indeed generated in murine heart, highlighting an autocrine/paracrine action of CST in cardiac function.
To gain better insights into the mechanisms responsible for the antiadrenergic effect of 5 nM CST, we measured Ca2+ transients on electric-field-stimulated rat ventricular cardiomyocytes, in basal conditions and after Iso stimulation. The lack of any inhibitory effect of 5 nM WT-CST on isolated ventricular cells led us to hypothesize that CST does not act directly on cardiomyocytes but, rather, on other cell types contained alongside them in cardiac tissue, presumably endothelial cells. We have validated our hypothesis by pharmacological experiments on papillary muscle, where pretreatment with wortmannin, L-NNA, ODQ, or Triton X-100 significantly reduced the antiadrenergic effect of CST.
The results obtained on BAE-1 cells reinforced the evidence of a CST-mediated endothelial NO production by means of a Ca2+-independent, Akt-dependent eNOS phosphorylation. This pathway in endothelial cells has been previously reported for insulin, insulin-like growth factor-1, and oestrogens,37–40 and recently confirmed for the other CgA-derived peptide VS-1.23,24 Moreover, we recently showed24 that the VS-1-mediated eNOS activation involves a proteoglycans-PI3-K-dependent caveolae endocytosis as the initiating factor, providing an appealing novel signalling pathway expected for receptor-orphan peptides, like CST, with membrane-interacting properties.
In summary, the above findings provide the mechanisms underlying both the cardiac inotropic and the vasodilator effects of CST observed in different preparations.10,41 Although the vasodilator effect exerted in vivo by CST has been attributed to histamine release and H1 receptors stimulation,17 CST-induced NO release from endothelial cells may act in concert to execute this effect.
4.1. Cardiac profile of CST variants
Recent reports demonstrated that Gly/Ser heterozygotes display profound changes in autonomic activity, including increased parasympathetic and decreased sympathetic indices, coupled with reduced catecholamine excretion. Moreover, the Gly364Ser variant is associated with a decrease in diastolic blood pressure, particularly in males.6 Because P370L-CST variation is rare (0.6%) and occurs in individuals from African ancestry, its association with cardiovascular function is yet to be established.22,42 In the present study, we found that the variant P370L-CST increased NO production and exerted an anti-adrenergic effect comparable to that induced by WT-CST, while G364S-CST was completely ineffective. In agreement with those previously reported for the isolated heart,10 our results confirm the physiological significance of heterologous human CST variants, showing that they display a different efficacy in counteracting the positive inotropic effect of Iso, thus providing a cardiotropic counterpart of the antihypertensive effects shown by the peptide. Moreover, as it has been shown that Gly/Ser polymorphism modifies an highly conserved site in mammalian CST sequence,43 we hypothesize that the amino acid substitution engenders subtle structural/conformational alterations of CST, responsible for its variation in subcellular signalling and therefore in potency.22
4.2. Conclusions and perspectives
Taken together, our data strongly support the hypothesis that CST, together with the other CgA fragments (i.e. the N-terminal CgA1–76, VS-1), may be a component of an endocrine/paracrine regulation of cardiac function.2 Recent findings suggest that, besides acting as an endogenous regulator of catecholamine secretion from chromaffin cells,15 locally derived CST may regulate cardiac function when specific stimulus-induced proteolytic activation could generate CgA fragments. Of particular interest is the fact that the previously discovered antihypertensive modulatory action of CST appears also associated with a direct powerful counterexcitatory cardiac action. The cardiac inhibitory action of CST may be important not only for the normal heart function, but also under pathological conditions characterized by adrenosympathogenic overactivation.44 In this perspective, CST acquires biomedical interest in those physiopathological conditions characterized by prolonged and excessive adrenergic activity, e.g. heart failure and hypertensive cardiomyopathy. On the other hand, our study provide further insights into the mechanism(s) of action of this peptide promoting the release of NO from endothelial cells by means of a Ca2+-independent mechanism and involving Akt-dependent phosphorylation of eNOS.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Conflict of interest: none declared.
Funding
The authors were supported by funding from Compagnia di S. Paolo, Torino, Italy, by the National Institute of Cardiovascular Research (INRC), Bologna, Italy, and from the Italian Ministry of the University and Research (MIUR), Progetti di Rilevanza Nazionale (PRIN, 2008) (B.T.). S.K.M. was supported by grants from the Veterans Affairs and the National Institutes of Health.
Supplementary Material
References
- 1.Helle KB, Corti A, Metz-Boutigue MH, Tota B. The endocrine role for chromogranin A: a prohormone for peptides with regulatory properties. Cell Mol Life Sci. 2007;64:2863–2886. doi: 10.1007/s00018-007-7254-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tota B, Cerra MC, Gattuso A. Catecholamines, cardiac natriuretic peptides and chromogranin A: evolution and physiopathology of a “whip-brake” system of the endocrine heart. J Exp Biol. 2010;213:3081–3103. doi: 10.1242/jeb.027391. [DOI] [PubMed] [Google Scholar]
- 3.Pieroni M, Corti A, Tota B, Curnis F, Angelone T, Colombo B, et al. Myocardial production of chromogranin A in human heart: a new regulatory peptide of cardiac function. Eur Heart J. 2007;28:1117–1127. doi: 10.1093/eurheartj/ehm022. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor DT, Deftos LJ. Secretion of chromogranin A by peptide-producing endocrine neoplasms. N Engl J Med. 1986;314:1145–1151. doi: 10.1056/NEJM198605013141803. [DOI] [PubMed] [Google Scholar]
- 5.Mahapatra NR, O'Connor DT, Vaingankar SM, Hikim AP, Mahata M, Ray S, et al. Hypertension from targeted ablation of chromogranin A can be rescued by the human ortholog. J Clin Invest. 2005;115:1942–1952. doi: 10.1172/JCI24354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rao F, Wen G, Gayen JR, Das M, Vaingankar SM, Rana BK, et al. Catecholamine release-inhibitory peptide catestatin (chromogranin A(352–372): naturally occurring amino acid variant Gly364Ser causes profound changes in human autonomic activity and alters risk for hypertension. Circulation. 2007;15:2271–2281. doi: 10.1161/CIRCULATIONAHA.106.628859. [DOI] [PubMed] [Google Scholar]
- 7.Jansson AM, Røsjø H, Omland T, Karlsson T, Hartford M, Flyvbjerg A, et al. Prognostic value of circulating chromogranin A levels in acute coronary syndromes. Eur Heart J. 2009;30:25–32. doi: 10.1093/eurheartj/ehn513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ceconi C, Ferrari R, Bachetti T, Opasich C, Volterrani M, Colombo B, et al. Chromogranin A in heart failure; a novel neurohumoral factor and a predictor for mortality. Eur Heart J. 2002;23:967–974. doi: 10.1053/euhj.2001.2977. [DOI] [PubMed] [Google Scholar]
- 9.Omland T, Dickstein K, Syversen U. Association between plasma chromogranin A concentration and long-term mortality after MI. Am J Med. 2003;114:25–30. doi: 10.1016/s0002-9343(02)01425-0. [DOI] [PubMed] [Google Scholar]
- 10.Angelone T, Quintieri AM, Brar BK, Limchaiyawat PT, Tota B, Mahata SK, et al. The antihypertensive chromogranin A peptide catestatin acts as a novel endocrine/paracrine modulator of cardiac inotropism and lusitropism. Endocrinology. 2008;149:4780–4793. doi: 10.1210/en.2008-0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mahata SK, Mahata M, Fung MM, O'Connor DT. Catestatin: a multifunctional peptide from chromogranin A. Regul Pept. 2010;162:33–43. doi: 10.1016/j.regpep.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Connor DT, Kailasam MT, Kennedy BP, Ziegler MG, Yanaihara N, Parmer RJ. Early decline in the catecholamine release-inhibitory peptide catestatin in humans at genetic risk of hypertension. J Hypertens. 2002;20:1335–1345. doi: 10.1097/00004872-200207000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Mahata SK, O'Connor DT, Mahata M, Yoo SH, Taupenot L, Wu H, et al. Novel autocrine feedback control of catecholamine release. A discrete chromogranin a fragment is a noncompetitive nicotinic cholinergic antagonist. J Clin Invest. 1997;100:1623–1633. doi: 10.1172/JCI119686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mahata SK, Mahata M, Wakade AR, O'Connor DT. Primary structure and function of the catecholamine release inhibitory peptide catestatin (chromogranin A(344-364)): identification of amino acid residues crucial for activity. Mol Endocrinol. 2000;14:1525–1535. doi: 10.1210/mend.14.10.0531. [DOI] [PubMed] [Google Scholar]
- 15.Mahata SK, Mahata M, Wen G, Wong WB, Mahapatra NR, Hamilton BA, et al. The catecholamine release-inhibitory “catestatin” fragment of chromogranin A: naturally occurring human variants with different potencies for multiple chromaffin cell nicotinic cholinergic responses. Mol Pharmacol. 2004;66:1180–1191. doi: 10.1124/mol.104.002139. [DOI] [PubMed] [Google Scholar]
- 16.Herrero CJ, Alés E, Pintado AJ, López MG, García-Palomero E, Mahata SK, et al. Modulatory mechanism of the endogenous peptide catestatin on neuronal nicotinic acetylcholine receptors and exocytosis. J Neurosci. 2002;22:377–388. doi: 10.1523/JNEUROSCI.22-02-00377.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kennedy BP, Mahata SK, O'Connor DT, Ziegler MG. Mechanism of cardiovascular actions of the chromogranin A fragment catestatin in vivo. Peptides. 1998;19:1241–1248. doi: 10.1016/s0196-9781(98)00086-2. [DOI] [PubMed] [Google Scholar]
- 18.Fung MM, Salem RM, Mehtani P, Thomas B, Lu CF, Perez B, et al. Direct vasoactive effects of the chromogranin A (CHGA) peptide catestatin in humans in vivo. Clin Exp Hypertens. 2010;32:278–287. doi: 10.3109/10641960903265246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gayen JR, Gu Y, O'Connor DT, Mahata SK. Global disturbances in autonomic function yield cardiovascular instability and hypertension in the chromogranin A null mouse. Endocrinology. 2009;150:5027–5035. doi: 10.1210/en.2009-0429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dev NB, Gayen JR, O'Connor DT, Mahata SK. Chromogranin A and the autonomic system: decomposition of heart rate variability and rescue by its catestatin fragment. Endocrinology. 2010;151:2760–2768. doi: 10.1210/en.2009-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Penna C, Alloatti G, Gallo MP, Cerra MC, Levi R, Tullio F, et al. Catestatin improves post-ischemic left ventricular function and decreases ischemia/reperfusion injury in heart. Cell Mol Neurobiol. 2010;30:1171–1179. doi: 10.1007/s10571-010-9598-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wen G, Mahata SK, Cadman P, Mahata M, Ghosh S, Mahapatra NR, et al. Both rare and common polymorphisms contribute functional variation at CHGA, a regulator of catecholamine physiology. Am J Hum Genet. 2004;74:197–207. doi: 10.1086/381399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gallo MP, Levi R, Ramella R, Brero A, Boero O, Tota B, et al. Endothelium-derived nitric oxide mediates the antiadrenergic effect of human vasostatin-1 in rat ventricular myocardium. Am J Physiol Heart Circ Physiol. 2007;292:H2906–H2912. doi: 10.1152/ajpheart.01253.2006. [DOI] [PubMed] [Google Scholar]
- 24.Ramella R, Boero O, Alloatti G, Angelone T, Levi R, Gallo MP. Vasostatin 1 activates eNOS in endothelial cells through a proteoglycan-dependent mechanism. J Cell Biochem. 2010;110:70–79. doi: 10.1002/jcb.22510. [DOI] [PubMed] [Google Scholar]
- 25.Imbrogno S, Garofalo F, Cerra MC, Mahata SK, Tota B. The catecholamine release-inhibitory peptide catestatin (chromogranin344-364) modulates myocardial function in fish. J Exp Biol. 2010;213:3636–3643. doi: 10.1242/jeb.045567. [DOI] [PubMed] [Google Scholar]
- 26.Takiyyuddin MA, Cervenka JH, Sullivan PA, Pandian MR, Parmer RJ, Barbosa JA, et al. Is physiologic sympathoadrenal catecholamine release exocytotic in humans? Circulation. 1990;81:185–195. doi: 10.1161/01.cir.81.1.185. [DOI] [PubMed] [Google Scholar]
- 27.Cerra MC, De Iuri L, Angelone T, Corti A, Tota B. Recombinant N-terminal fragments of chromogranin-A modulate cardiac function of the Langendorff-perfused rat heart. Basic Res Cardiol. 2006;101:43–52. doi: 10.1007/s00395-005-0547-2. [DOI] [PubMed] [Google Scholar]
- 28.Corti A, Mannarino C, Mazza R, Colombo B, Longhi R, Tota B. Vasostatins exert negative inotropism in the working heart of the frog. Ann NY Acad Sci. 2002;971:362–365. doi: 10.1111/j.1749-6632.2002.tb04497.x. [DOI] [PubMed] [Google Scholar]
- 29.Imbrogno S, Angelone T, Corti A, Adamo C, Helle KB, Tota B. Influence of vasostatins, the chromogranin A-derived peptides, on the working heart of the eel (Anguilla anguilla): negative inotropy and mechanism of action. Gen Comp Endocrinol. 2004;139:20–28. doi: 10.1016/j.ygcen.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 30.Kruger PG, Mahata SK, Helle KB. Catestatin (CgA344-364) stimulates rat mast cell release of histamine in a manner comparable to mastoparan and other cationic charged neuropeptides. Regulatory Peptides. 2003;114:29–35. doi: 10.1016/s0167-0115(03)00069-7. [DOI] [PubMed] [Google Scholar]
- 31.Hattori Y. Cardiac histamine receptors: their pharmacological consequences and signal transduction pathways. Methods Find Exp Clin Pharmacol. 1999;21:123–131. doi: 10.1358/mf.1999.21.2.529239. [DOI] [PubMed] [Google Scholar]
- 32.Matsuda N, Jesmin S, Takahashi Y, Hatta E, Kobayashi M, Matsuyama K, et al. Histamine H1 and H2 receptor gene and protein levels are differentially expressed in the hearts of rodents and humans. J Pharmacol Exp Ther. 2004;309:786–795. doi: 10.1124/jpet.103.063065. [DOI] [PubMed] [Google Scholar]
- 33.Helle KB. The granin family of uniquely acidic proteins of the diffuse neuroendocrine system: comparative and functional aspects. Biol Rev. 2004;79:769–794. doi: 10.1017/s146479310400644x. [DOI] [PubMed] [Google Scholar]
- 34.Steiner HJ, Weiler R, Ludescher C, Schmid KW, Winkler H. Chromogranins A and B are co-localized with atrial natriuretic peptides in secretory granules of rat heart. J Histochem Cytochem. 1990;38:845–850. doi: 10.1177/38.6.2139887. [DOI] [PubMed] [Google Scholar]
- 35.Weiergraber M, Pereverzev A, Vajna R, Henry M, Schramm M, Nastainczyk W, et al. Immunodetection of alpha1E voltage-gated Ca2+ channel in chromogranin-positive muscle cells of rat heart and in distal tubules of human kidney. J Histochem Cytochem. 2000;48:807–819. doi: 10.1177/002215540004800609. [DOI] [PubMed] [Google Scholar]
- 36.Biswas N, Curello E, O'Connor DT, Mahata SK. Chromogranin/secretogranin proteins in murine heart: myocardial production of chromogranin A fragment catestatin (Chga(364-384)) Cell Tissue Res. 2010;342:353–361. doi: 10.1007/s00441-010-1059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Aktdependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 38.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, et al. Regulation of endothelium derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartell NA, Archer HE, Bailey CJ. Insulin-stimulated endothelial nitric oxide release is calcium independent and mediated via protein kinase B. Biochem Pharmacol. 2005;69:781–790. doi: 10.1016/j.bcp.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 40.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–774. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 41.Imbrogno S, Garofalo F, Cerra MC, Mahata SK, Tota B. The catecholamine release-inhibitory peptide catestatin (chromogranin A344-364) modulates myocardial function in fish. J Exp Biol. 2010;213:3636–3643. doi: 10.1242/jeb.045567. [DOI] [PubMed] [Google Scholar]
- 42.Biswas N, Vaingankar SM, Mahata M, Das M, Gayen JR, Taupenot L, et al. Proteolytic cleavage of human chromogranin a containing naturally occurring catestatin variants: differential processing at catestatin region by plasmin. Endocrinology. 2008;149:749–757. doi: 10.1210/en.2007-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsigelny I, Mahata SK, Taupenot L, Preece NE, Mahata M, Khan I, et al. Mechanism of action of chromogranin A on catecholamine release: molecular modeling of the catestatin region reveals a beta-strand/loop/beta-strand structure secured by hydrophobic interactions and predictive of activity. Regul Pept. 1998;77:43–53. doi: 10.1016/s0167-0115(98)00040-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samuels MA. The heart-brain connection. Circulation. 2007;116:77–84. doi: 10.1161/CIRCULATIONAHA.106.678995. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.