Abstract
Background. Filamentous fungi of the genera Aspergillus and Fusarium are major causes of corneal ulcers in the United States and in the developing world and result in significant visual impairment and blindness.
Methods. RNA was extracted from 110 patients with corneal ulcers in southern India within 1 week of infection with either Fusarium solani or Aspergillus flavus, and gene expression was determined by quantitative polymerase chain reaction. Posttransplant corneas from later stage disease (>2 weeks after infection) were also examined.
Results. Expression of Dectin-1, Toll-like receptor 2 (TLR2), TLR4, TLR9, and NOD-like receptor protein (NLRP)3 messenger RNA was elevated >1000-fold compared with uninfected donor corneas, whereas Dectin-2 was constitutively expressed in uninfected corneas. Furthermore, interleukin 1β (IL-1β) expression was elevated >1000-fold, whereas IL-1α expression was not increased. Expression of IL-8, IL-17, and tumor necrosis factor α was also elevated. CD3+and CD4+ T cells were detected in infected posttransplant corneas. Expression of IL-17 and interferon γ was elevated but not that of IL-4. There were no significant differences in the host response between Aspergillus- and Fusarium-infected corneas at any time point.
Conclusions. There is a common innate and adaptive immune response to these filamentous fungi, which includes the generation of T-helper 1 and T-helper 17 cells.
Fungal keratitis is an important cause of blindness and visual impairment worldwide. In the United States and other industrialized countries, contact lens wear is the primary risk factor for fungal keratitis, as illustrated by several hundred cases in the United States, Western Europe, and Singapore during the 2005–2006 outbreak of Fusarium keratitis [1–3]. Although the incidence of fungal keratitis dropped after withdrawal of the lens care solution associated with the outbreak [2], the prevalence due to trauma or contact lens wear has remained consistent in the United States, especially in southeastern regions [4].
Contact-lens–related fungal keratitis is associated with biofilm formation [5], whereas in trauma-related disease, corneas are infected as a result of ocular surface trauma caused by plant material, insects, or branches contaminated with fungal spores [6–8]. Once the corneal epithelial barrier is breached, the conidia germinate and penetrate throughout the corneal stroma. Depending on the inoculum and the time between infection and the patient receiving antifungal treatment (usually topical natamycin or voriconazole), infected individuals will either recover or undergo corneal transplantation [9]. In China and India, the major risk factor is agricultural work; consequently, the incidence of fungal keratitis increases during the harvest season, when there is greater exposure to airborne soil and contaminated plant material [6–8, 10, 11]. Once in the corneal stroma, Aspergillus and Fusarium conidia germinate, and hyphae penetrate the corneal stroma, causing corneal ulceration, severe pain, and visual impairment.
In contrast to pulmonary and systemic fungal infections, which occur primarily in immunocompromised individuals such as stem cell transplant recipients [12–15], patients with fungal keratitis are immunocompetent. In addition, fungal infections of the cornea present a unique opportunity to examine infected tissues at very early stages of infection because infected individuals experience severe pain and visual impairment and generally know when they experienced ocular surface trauma, which is the likely time of infection. Because treatment failure is also common, patients undergo corneal transplantation, thereby providing infected tissue from a later time point after infection.
In the present study, we therefore examined the host response to these pathogenic fungi in infected corneal ulcers within days of infection and in infected posttransplant corneas at later time points after infection. Our findings demonstrate that infected individuals mount a profound host response to Aspergillus and Fusarium hyphae that is characterized by infiltration of neutrophils, macrophages, and T cells and by expression of proinflammatory, chemotactic, and regulatory cytokines.
METHODS
Human Subjects
The protocol for obtaining corneal ulcer scrapings and posttransplant infected corneas removed at the time of surgery was reviewed and approved by the ethics committee of the Aravind Medical Research Foundation. The research aims and methodology are thoroughly explained to the patients, and the samples are collected after obtaining informed consent. Patients with acute or chronic systemic illness or with any form of immunosuppression or topical steroid therapy were excluded from this study. All studies on patient material were performed in India.
Characterization of Fungal Species
The species of fungal isolates were identified using a combination of morphology and DNA sequence methods. Aspergillus flavus was initially recognized by the production of abundant yellow-green, biseriate conidiophores, and Fusarium solani species complex members were recognized by the shape of macroconidia and the production of microconidia from long monophialidic conidiophores. DNA sequences of the internal transcribed spacer region of the ribosomal RNA gene repeat (A. flavus) and partial translation elongation factor 1-α (F. solani complex) were determined using standard methods [16, 17].
RNA Extraction, Complementary DNA Conversion, and Quantitative Polymerase Chain Reaction Analysis
Corneal scrapings from the patient’s ulcers were homogenized in TRIzol (Invitrogen, Bangalore, India) by means of a handheld homogenizer (Labware Scientific, Kolkata, India), and total RNA was extracted from corneal tissue samples according to the manufacturer’s directions, followed by DNase treatment (Invitrogen). The quality of RNA was checked by agarose gel electrophoresis, and samples with a 260/280 ratio of >1.8 were used to generate complementary DNA (cDNA).
The cDNA was generated using the SuperScript First Stand synthesis system (Invitrogen) by means of standard methods. Samples of cDNA were then digested with RNaseH (Invitrogen) for 20 minutes at 37°C, and quantitative polymerase chain reaction (PCR) was performed using the SYBR green system (Applied Biosystems, Carlsbad, CA). Primer sequences were designed using NCBI Primer BLAST or downloaded from Primer bank (Table 1; online only) and synthesized at either Bioserve India (Hyderabad, India) or Integrated DNA Technologies (San Diego, CA). Universal PCR conditions were utilized for cDNA amplification for all primer sets. Melting curve analysis was performed to confirm specific gene amplification, and PCR product sizes were confirmed by agarose gel electrophoresis.
The quantification cycle of the target gene was normalized using GAPDH (which was consistent for all samples), and the fold change with respect to noninfected donor corneas was calculated using the 2−ΔΔCt method. Data are therefore presented as log of relative gene expression, as originally described by Livak and Schmittgen [18].
Characterization of Host Cells in Corneal Tissues
Infected corneal ulcer material was spread on a standard microscope slide and stained with the modified Wrights–Geimsa solutions (Diff-Quik). Infected corneas obtained after transplantation were fixed in 4% paraformaldehyde and paraffin-embedded, and 5-μm sections were stained with periodic acid Schiff and Gomori-Methanamine silver (GMS) according to standard protocols. For immunohistochemistry, paraffin-embedded 5-μm sections of infected corneas were deparaffinized with xylene and ethanol gradient methods and incubated for 10 minutes at room temperature with 3% freshly prepared hydrogen peroxide solution to block endogenous peroxidase activity. Antigen retrieval was performed by 3 cycles of microwaving with slides submerged in citrate buffer (sodium citrate dehydrate, 2.94 g/L; pH, 6.0) for 6 minutes at 50% power. The slides were then washed with phosphate-buffered saline (PBS) with 0.05% Tween-80 (PBS-T), blocked with 5% horse serum in PBS-T for 30 minutes, and incubated with mouse monoclonal antibodies with specificity for human neutrophil elastase, macrophage CD68, or human CD3 or CD4 (Abcam, Cambridge, MA). All primary antibodies were used at a 1:50 dilution in PBS-T and incubated overnight at 4°C.
The sections were then washed, incubated for 1 hour at room temperature with a secondary antimouse antibody (ImmPRESS reagent kit; Vector labs), washed with PBS, and incubated with 3,3′-diaminobenzidine (Vector labs) in the dark for 2–10 minutes. The reaction was stopped with double distilled H2O, and sections were counterstained with hematoxylin. Sections were dehydrated in ethanol gradients, dipped in xylene, mounted using DPX mounting medium (Merck), and dried overnight before examination. For anti-CD4 antibody staining, sections were incubated with Alexafluor-488–conjugated antimouse immunoglobulin G antibody (Invitrogen) and washed with PBS-T; VectaShield with 4′,6-diamidino-2-phenylindole (DAPI; Vector Labs) was used as the mounting medium.
To detect β-glucan, corneal sections were incubated with primary mouse antifungal β-glucan immunoglobulin M (BF-Div; Biothera/Brown University) for 1 hour at 37°C, followed by incubation for 1 hour at 37°C with 1 mg/mL alexafluor-488–conjugated rabbit-antimouse immunoglobulin M (Invitrogen). Slides were washed in PBS-T and mounted with VectaShield, and sections were imaged by fluorescence microscopy.
Statistical Analysis
Differences in gene expression between normal, uninfected donor corneas and infected corneas were calculated by 1-way analysis of variance, and differences between Aspergillus- and Fusarium-infected corneal tissue were calculated by the unpaired t test with the use of GraphPad Prism software. Statistical significance was defined as P < .05.
RESULTS
Characteristics of the Study Population
Patients with corneal ulcers are routinely treated at the Aravind Eye Hospital, which is a primary care facility in Madurai, Tamil Nadu, India. These patients generally present with severe pain and visual impairment within days of experiencing ocular trauma. Although bacteria are frequently the cause of corneal ulcers, the majority of ulcers are caused by Aspergillus or Fusarium species and are associated with corneal injury from plant or soil material or from other causes such as insects, sticks, and cattle tails [6, 19]. In the present study, which was undertaken from January through March 2010, samples from patients with corneal ulcers but without systemic illness or immunosuppressive therapy were selected. Ethical clearance was obtained from the internal institutional review board at Aravind Eye Hospital, and informed consent was obtained from all participants.
Corneal ulcer scrapings were cultured, and morphological analysis identified 55 patients infected with Fusarium species and 55 patients infected with A. flavus (Table 1). Subsequent examination of these isolates by sequence analysis confirmed the identity of A. flavus and showed that Fusarium species were almost exclusively members of the F. solani family complex [16, 17].
Table 1.
Characteristics of Patients Whose Corneal Samples Were Recovered From January Through March 2010
| Characteristic | Group IA |
Group IB |
Group II |
|||
| Fusarium sp | A. flavus | Fusarium sp | A. flavus | Fusarium sp | A. flavus | |
| Age, years | ||||||
| Range | 20–70 | 27–70 | 21–80 | 14–70 | 30–75 | 30–64 |
| Mean (SD) | 46.30 (15.70) | 50.10 (14.02) | 52.77 (14.60) | 50.05 (15.75) | 48.80 (15.10) | 46.30 (10.41) |
| Sex | ||||||
| Male | 17 (73.91) | 12 (57.14) | 12 (54.55) | 11 (57.89) | 5 (50.00) | 7 (70.00) |
| Female | 6 (26.09) | 9 (42.86) | 10 (45.45) | 8 (42.11) | 5 (50.00) | 3 (30.00) |
| Total | 23 (100.00) | 21 (100.00) | 22 (100.00) | 19 (100.00) | 10 (100.00) | 10 (100.00) |
| Size of ulcer, mm2 | ||||||
| <5 | 2 (8.70) | 1 (4.76) | 2 (9.09) | NA | NA | NA |
| 5–10 | 5 (21.74) | 4 (19.05) | 9 (40.91) | 3 (15.79) | NA | NA |
| 10–14 | 10 (43.48) | 8 (38.10) | 4 (18.18) | 9 (47.37) | 1 (10.00) | 2 (20.00) |
| >14 | 6 (26.09) | 8 (38.10) | 7 (31.82) | 7 (36.84) | 9 (90.00) | 8 (80.00) |
| Depth of ulcer | ||||||
| Superficial | 4 (17.39) | 2 (9.52) | 5 (22.73) | 3 (15.79) | NA | NA |
| Mild | 4 (17.39) | 6 (28.57) | 6 (27.27) | 7 (36.84) | 1 (10.00) | 2 (20.00) |
| Deep | 15 (65.22) | 13 (61.90) | 11 (50.00) | 9 (47.37) | 9 (90.00) | 8 (80.00) |
| Hypopyon presence | ||||||
| Yes | 19 (82.61) | 16 (76.19) | 12 (54.55) | 10 (52.63) | 9 (90.00) | 9 (90.00) |
| No | 4 (17.39) | 5 (23.81) | 10 (45.45) | 9 (47.37) | 1 (10.00) | 1 (10.00) |
| Clinical outcome | ||||||
| Healed | 7 (30.43) | 4 (19.05) | 6 (27.27) | 5 (26.32) | NA | NA |
| Treatment failure | 4 (17.39) | 8 (38.10) | 6 (27.27) | 7 (36.84) | 10 (100.00) | 10 (100.00) |
| Lost to follow-up | 12 (52.17) | 10 (47.62) | 10 (45.45) | 7 (36.84) | NA | NA |
NOTE. Data are no. (%) of patients, unless otherwise indicated. Patients (n = 85) in groups IA and IB had corneal ulcers and presented at the clinic within 1–2 weeks after infection; corneal scrapings from the ulcer were used in the present study. Patients (n = 20) in group II had undergone corneal transplants ≥2 weeks after infection, and infected posttransplant corneas were used in the present study. Ten donor corneas from individuals with no infection or inflammation were obtained from the Aravind Hospital’s Eye Bank (mean age, 61 years). A. flavus, Aspergillus flavus; Fusarium sp, Fusarium species; NA, not applicable.
Patients in groups IA and IB (n = 85) presented at the clinic between 3 and 7 days after infection, and corneal ulcer scrapings were used for analysis, whereas group II patients (n = 20) had been infected for >2 weeks and had undergone corneal transplantation; therefore, infected posttransplant corneas were used in the present study. All patients were 46–53 years old, and slightly more men than women were infected. The size and depth of ulcers among patients in groups IA and IB ranged from <5 to >14 mm in diameter, and the majority of patients showed hypopyon formation, which is accumulation of neutrophils in the anterior chamber. Among patients in groups IA and IB, 7%–38% did not heal, although 36%–52% of patients did not appear for a follow-up examination. All patients in group II experienced treatment failure. Control tissues were from corneal donors with a mean age of 61.70 years (+/-, 6.40 years) who died of natural causes and who had no history of corneal infection or other systemic disease.
Clinical Appearance and Cellular Infiltration in Early-Stage Aspergillus and Fusarium Keratitis
Figure 1 shows representative corneal ulcers caused by either A. flavus or F. solani. The patient with A. flavus keratitis shown is a 47-year-old woman with a central corneal ulcer and hypopyon formation, who experienced a foreign body trauma to the ocular surface while working on a farm and who presented at the clinic 1 week after infection. Her ulcer did not respond to treatment, and she successfully underwent corneal transplantation 2 weeks later. The patient with F. solani keratitis shown is a 55-year-old woman with a perforated ulcer, whose cornea was scratched by a sharp leaf and who appeared at the clinic 10 days later. She also underwent a corneal transplantation 2 weeks later, although the transplanted cornea was rejected.
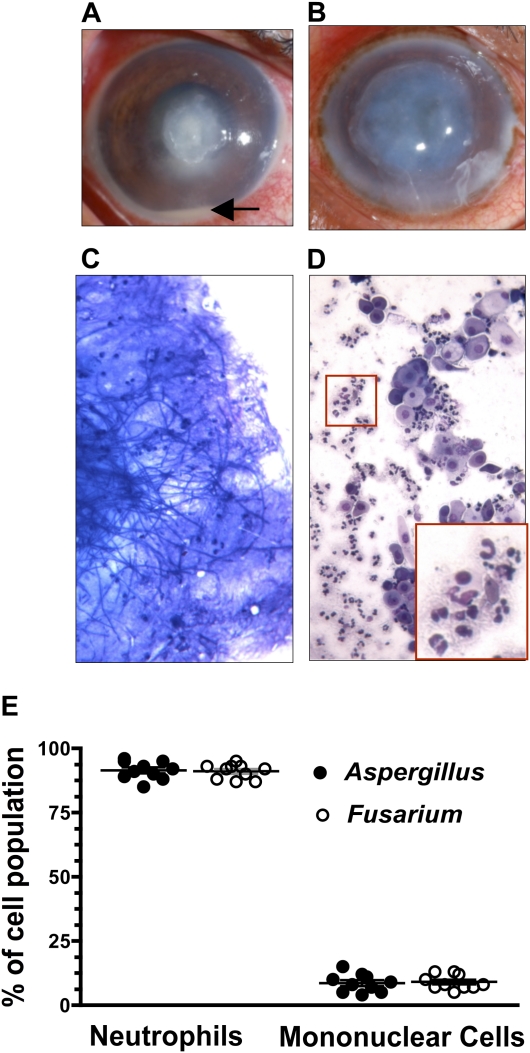
Figure 1.
Cellular composition of corneal ulcers from patients with fungal keratitis. Shown are representative corneal ulcers caused by Aspergillus flavus (A) or by Fusarium solani (B) with the arrow indicating accumulation of neutrophils in the anterior chamber (original magnification ×10). Ulcerative tissue was fixed and stained with Diff-Quik, and representative images show A. flavus hyphae (C) and the cellular infiltrate (D) in infected corneas (original magnification ×400). The inset in panel D shows the typical nuclear morphology of neutrophils. The percentages of neutrophils and mononuclear cells were determined by counting 100 cells from 10 patients with Aspergillus infection and 10 patients with Fusarium infection (E).
To identify infiltrating cells in the corneal ulcer, a scraping was stained with Diff-Quik and examined by light microscopy. Hyphae were readily detected in corneal ulcers infected with A. flavus (Figure 1) and F. solani (not shown). Neutrophils comprised 95% of infiltrating cells, with 5% being mononuclear cells, including epithelial cells and monocytes (Figure 1).
Expression of Pathogen Recognition Receptors and Inflammasome Genes in Early-Stage Aspergillus and Fusarium Keratitis
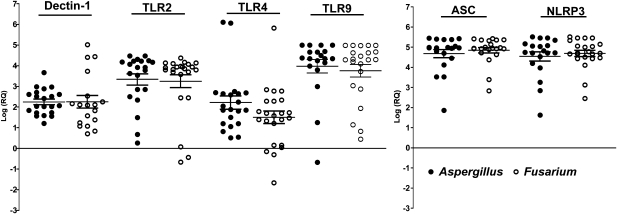
To determine the expression of pathogen recognition receptors in early-stage fungal keratitis, RNA was extracted from 85 corneal ulcer scrapings within 1 week of trauma (group I) and reverse transcribed; quantitative PCR was then performed. ΔCt data were calculated using GAPDH (which was consistent for all samples), and the fold change with respect to the mean of 10 noninfected donor corneas was derived using the 2−ΔΔCt method [18]. Figure 2 shows that Dectin-1 expression was elevated a mean of 500-fold in Aspergillus- and Fusarium-infected corneas compared with donor corneas; the level of Toll-like receptor 4 (TLR4) was elevated 500-fold, and levels of TLR2, TLR9, adaptor protein apoptosis speck protein with caspase recruitment (ASC), and NOD-like receptor protein (NLRP)3 were elevated >1000-fold above the levels in uninfected corneas. There were no significant differences in the expression of any of these genes between Aspergillus- and Fusarium-infected corneas.
Figure 2.
Expression of pathogen recognition receptors in corneal ulcers from patients with fungal keratitis. RNA was extracted from corneal ulcerative tissue, reverse transcribed, and processed for quantitative polymerase chain reaction. Data points represent individual patients with Aspergillus or Fusarium keratitis, and the values are presented as the log of relative gene expression (log[RQ]) relative to uninfected donor corneas calculated using the 2−ΔΔCt method described in the text. There were no significant differences in gene expression between Aspergillus-infected corneas (closed circles) and Fusarium-infected corneas (P > .05) (open circles). TLR, toll-like receptor.
ΔCt values for donor corneas and fungal-infected corneal ulcer material are shown in Supplementary Figure 1. These data show that expression of these pathogen recognition molecules and inflammasome proteins in donor corneas (high ΔCt value) was low compared with corneal ulcer material (low ΔCt value) from infected corneas. Supplementary Figure 1 also shows that Dectin-2 expression was elevated (low ΔCt value) in both donor and infected corneas, with no significant differences, which indicates constitutive expression of this receptor.
Differential Expression of Proinflammatory and Chemotactic Cytokines in Early-Stage Fungal Keratitis
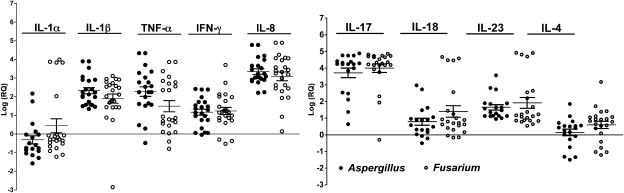
Given the elevated expression of NLRP3 and ASC, we examined expression of interleukin 1β (IL-1β) and other cytokines associated with neutrophil recruitment and activation. As shown in Figure 3, IL-1β transcripts in group IA were elevated a mean of 100-fold above the level in uninfected corneas, whereas IL-1α expression was not upregulated. Tumor necrosis factor α (TNF-α) and interferon γ (IFN-γ) expression levels were elevated 100-fold and 10-fold, respectively, whereas that of IL-8 was upregulated 1000-fold.
Figure 3.
Expression of proinflammatory, regulatory, and chemotactic cytokines in corneal ulcers. Q-PCR analysis of corneal ulcerative tissue was performed as described in Methods. Data points represent individual patients infected with Aspergillus or Fusarium and values were calculated as described above. There were no significant differences in gene expression between Aspergillus- (closed circles) and Fusarium- (open circles) infected corneas (P > .05). IFN, interferon; IL, interleukin; TNF, tumor necrosis factor; log(RQ); log of relative gene expression.
Consistent with the Dectin-1 finding, we also found that IL-17 expression in Aspergillus- and Fusarium-infected corneas was elevated 2000-fold above that in control corneas (Figure 3). In addition, IL-18 and IL-23 transcripts were elevated by 50-fold and 100-fold, respectively, whereas IL-4 expression was not increased above that of control corneas. As with expression of pathogen recognition receptors, there were no significant differences in cytokine expression between Aspergillus- and Fusarium-infected corneas, indicating that both genera of pathogenic fungi stimulate production of chemotactic and proinflammatory cytokines. Supplementary Figure 1 shows ΔCt values, confirming that IL-1α and IL-4 expression was low in both donor and infected tissues, whereas expression of all other cytokines was significantly elevated in infected compared with donor corneal tissues.
Expression of β-Glucan and Characterization of the Cellular Infiltrate in Posttransplant Corneas Infected With Aspergillus or Fusarium
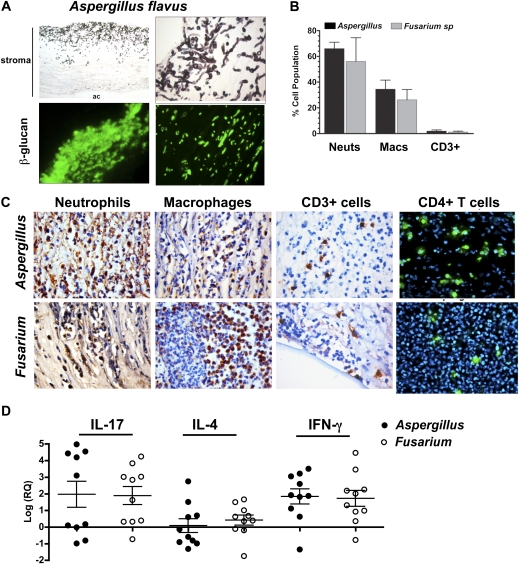
Because Dectin-1 recognizes β-glucan on germinating conidia and hyphae [20], we next examined β-glucan expression in posttransplant infected corneas. Five-micron corneal sections were either stained with GMS or immunostained with an antibody that recognizes 1,3-β-glucan and 1,6-β-glucan. Figure 4 shows extensive fungal growth in GMS-stained corneas infected with A. flavus and β-glucan expression in infected corneas. There was no expression of β-glucan in normal donor corneas (data not shown).
Figure 4.
β-glucan and cellular infiltration in infected posttransplant corneas. A, Five-micron sections of Aspergillus flavus–infected corneas stained with Gomori-Methanamine silver to identify fungal hyphae or immunostained with antibodies to β-glucan (Dectin-1 ligand) (original magnification ×100 in upper left panel, ×400 in upper right and lower left panels, and ×1000 in lower right panel). Stroma and anterior chamber (ac) are indicated. B, Percentage of cell types (neutrophils [neuts] and macrophages [macs]) in Fusarium- and Aspergillus-infected corneas; 5 slides were examined for each stain, and 100 cells were counted on each section. C, Representative corneal sections of Aspergillus- and Fusarium-infected corneas immunostained with antibodies to neutrophils (elastase), macrophages (CD68), CD3, and CD4. DAPI (blue) showed the presence of cell nuclei in the CD4-stained sections, and 3,3′-diaminobenzidine and hematoxylin were used to visualize cells in other sections (original magnification ×400). D, Results of quantitative polymerase chain reaction performed on corneal tissue as described in the legend to Figure 2. Data points represent corneas from individual patients infected with Aspergillus or Fusarium in relation to uninfected donor corneas as described in the text. There were no significant differences in gene expression between Aspergillus- and Fusarium-infected corneas (P > .05). IFN, interferon; IL, interleukin; log(RQ), log of relative gene expression.
To examine infiltrating cells in fungal-infected posttransplant corneas, sections were immunostained with cell-specific antibodies. We found that 65%–75% of infiltrating cells were elastase-positive neutrophils, 25%–35% were CD68+ macrophages, and 3%–8% were CD3+ and CD4+ T cells, with no differences between Aspergillus- and Fusarium-infected tissues (Figure 4). Representative images are also shown.
To determine the cytokine response in later-stage fungal keratitis, RNA was extracted from posttransplant corneas infected with Aspergillus or Fusarium, and gene expression was determined by quantitative PCR. As shown in Figure 4, expression of IL-17 and IFN-γ in Aspergillus- and Fusarium-infected corneas was elevated a mean of 100-fold compared with normal donor corneas, whereas IL-4 expression was not increased. ΔCt values show significantly elevated IL-17 and IFN-γ but not IL-4 in infected corneas, compared with donor corneas, and IL-4 expression was low in both donor and infected corneas (Supplementary Figure 1). These findings, together with the results shown in Figure 4, are consistent with a T-helper 1 (Th1) and Th17 response, but not a Th2 response, in infected corneas.
DISCUSSION
Studies of family members and stem cell transplant recipients with the Tyr238X Dectin-1 early stop codon mutation have found that these individuals are more susceptible to mucocutaneous fungal infections, and blood mononuclear cells from these individuals have an impaired cytokine response to Candida albicans infections [21, 22]. Similarly, susceptibility to mucocutaneous candidiasis is associated with a point mutation in caspase recruitment domain (CARD9) [23], which is downstream of Dectin-1. Furthermore, stem cell transplant recipients with TLR4 polymorphisms were susceptible to invasive aspergillosis [24]. In the present study, we examined the expression of Dectin-1 and TLR4 in human tissue infected with Aspergillus or Fusarium.
We found that Dectin-1 expression is elevated in early-stage disease, which is consistent with our studies using a murine model of Aspergillus fumigatus keratitis in which Dectin-1 was found to mediate cytokine production, neutrophil recruitment, and fungal survival [25]. Murine models of pulmonary aspergillosis and of mucosal and systemic candidiasis also indicate that Dectin-1 regulates fungal survival and the outcome of disease [26, 27], although one study found no role for Dectin-1 in candidiasis [28].
In contrast to Dectin-1, Dectin-2 recognizes high mannose residues in the cell wall, responds to fungal hyphae rather than yeast, and functions together with Dectin-1 to induce IL-17 production [29–31]. In the present study, we found that IL-17 expression was greatly elevated in early-stage disease and that Dectin-2 expression was elevated in donor and infected corneas. Functional studies using mouse models of keratitis are currently underway to ascertain the role of Dectin-1 and Dectin-2 in IL-17 production and disease pathogenesis.
TLR4 expression was also elevated in Aspergillus- and Fusarium-infected corneas at early time points, which is consistent with the reported correlation between TLR4 polymorphisms and susceptibility to Aspergillus infections [24] and with our findings that TLR4 regulates Aspergillus and Fusarium clearance from mouse corneas [25, 32]. Although the mechanism has yet to be determined, TLR4 recognizes N-linked mannans [33], and we showed that TLR4-mediated fungal killing is independent of the lipopolysaccharide receptor, myeloid differentiation factor (MD)-2 [25], suggesting that N-linked mannans bind directly to TLR4.
We also found elevated TLR2 expression levels in infected corneal ulcer material. TLR2 contributes to inflammasome-mediated production of mature IL-1β in oral and systemic candidiasis [34, 35]. Furthermore, fungal activation of TLR2 on neutrophils induces an oxidative burst [36, 37] and contributes to fungal clearance in a murine model of C. albicans keratitis [38]. However, we found no role for TLR2 in murine models of Fusarium or Aspergillus keratitis [25, 32]; therefore, the significance of our current finding that TLR2 expression is elevated in infected human corneas has yet to be determined. TLR9 expression was also highly upregulated in Fusarium- and Aspergillus-infected human corneas, which may indicate a role for this receptor. In support of this notion, 2 recent reports demonstrated that TLR9 is recruited to phagosomes containing Aspergillus conidia, that Aspergillus DNA contains unmethylated CpG motifs, which activate TLR9 in vitro, and that TLR9−/− mice have an impaired response to Aspergillus in the lung [39, 40].
We anticipated that levels of proinflammatory and chemotactic cytokines would be elevated, and we found >1000-fold elevated expression of the neutrophil chemokine IL-8, which is consistent with the presence of neutrophils in corneal ulcers. In addition, expression of the proinflammatory cytokines IL-1β and TNF-α was elevated in infected corneas, whereas IL-1α expression was not elevated. In contrast to Dectin-2, this was not due to constitutively high expression in donor corneas but to low expression in all tissues examined. Neutrophils are also a possible source of IL-8 and other cytokines, and future studies will examine cytokine production by peripheral blood neutrophils incubated with live fungi.
Consistent with the observed increase in IL-1β transcripts, expression of the NLRP3 and ASC inflammasome proteins was greatly elevated, suggesting a role for inflammasomes in fungal keratitis. This finding is supported by reports showing an important role for IL-1R in murine models of Fusarium and Aspergillus keratitis [25, 32]. Although we found that TLR2, TLR4, and TLR5 are important in murine models of bacterial keratitis [41, 42], we have yet to determine whether these markers of inflammation are also upregulated in human bacterial keratitis.
The cytokine profile of late-stage disease showed elevated expression of IL-17 and IFN-γ but not IL-4, which together with the presence of 3%–8% CD3 and CD4 cells indicates that Th1 and Th17 cells are generated and recruited to the cornea. Although the presence of these T-cell subtypes in peripheral blood has yet to be determined, this finding is consistent with the predominant IFN-γ/T-cell responses reported for A. fumigatus in normal, asymptomatic individuals [43, 44], which likely occurs as a result of inhalation of these ubiquitous organisms [45]. Fusarium is also ubiquitous in agricultural environments; therefore, it seems reasonable to assume that most infected patients harbor memory T-cell responses to both Fusarium and Aspergillus prior to inoculation of spores into the corneal stroma, and that Th1- and Th17-like responses contribute to corneal pathology.
In conclusion, the results of the current study provide a direct characterization of the host response to pathogenic fungi in infected human tissues at early and later stages of disease in a population where it is endemic. These findings will allow us to examine specific host response genes with the use of animal models of fungal keratitis, and we may be able to identify potential targets for immune intervention that could regulate the severity of the host response to these organisms and the resulting visual impairment and blindness.
Supplementary Data
Supplementary data are available at The Journal of Infectious Diseases online.
Funding
This work was supported by the Indian Council of Medical Research (grant to P. L.); Alcon Laboratories (grant to P. L.); the Alcon Research Institute (award to E. P.); the National Eye Institute, National Institutes of Health (grants RO1 EY018612 and P30 EY011373 to E. P. and grant F31 EY019841 to S. M. L.); the Research to Prevent Blindness Foundation; and the Ohio Lions Eye Research Foundation.
Supplementary Material
References
- 1.Gaujoux T, Chatel MA, Chaumeil C, Laroche L, Borderie VM. Outbreak of contact lens-related Fusarium keratitis in France. Cornea. 2008;27:1018–21. doi: 10.1097/ICO.0b013e318173144d. [DOI] [PubMed] [Google Scholar]
- 2.Chang DC, Grant GB, O’Donnell K, et al. Multistate outbreak of Fusarium keratitis associated with use of a contact lens solution. JAMA. 2006;296:953–63. doi: 10.1001/jama.296.8.953. [DOI] [PubMed] [Google Scholar]
- 3.Khor WB, Aung T, Saw SM, et al. An outbreak of Fusarium keratitis associated with contact lens wear in Singapore. JAMA. 2006;295:2867–73. doi: 10.1001/jama.295.24.2867. [DOI] [PubMed] [Google Scholar]
- 4.Gower EW, Keay LJ, Oechsler RA, et al. Trends in fungal keratitis in the United States, 2001 to 2007. Ophthalmology. 2010;117:2263–7. doi: 10.1016/j.ophtha.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 5.Imamura Y, Chandra J, Mukherjee PK, et al. Fusarium and Candida albicans biofilms on soft contact lenses: model development, influence of lens type, and susceptibility to lens care solutions. Antimicrob Agents Chemother. 2008;52:171–82. doi: 10.1128/AAC.00387-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bharathi MJ, Ramakrishnan R, Meenakshi R, Padmavathy S, Shivakumar C, Srinivasan M. Microbial keratitis in South India: influence of risk factors, climate, and geographical variation. Ophthalmic Epidemiol. 2007;14:61–9. doi: 10.1080/09286580601001347. [DOI] [PubMed] [Google Scholar]
- 7.Bharathi MJ, Ramakrishnan R, Meenakshi R, Shivakumar C, Raj DL. Analysis of the risk factors predisposing to fungal, bacterial and Acanthamoeba keratitis in south India. Indian J Med Res. 2009;130:749–57. [PubMed] [Google Scholar]
- 8.Xie L, Zhong W, Shi W, Sun S. Spectrum of fungal keratitis in north China. Ophthalmology. 2006;113:1943–8. doi: 10.1016/j.ophtha.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Thomas PA. Fungal infections of the cornea. Eye (Lond) 2003;17:852–62. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Sun S, Jing Y, Han L, Zhang H, Yue J. Spectrum of fungal keratitis in central China. Clin Experiment Ophthalmol. 2009;37:763–71. doi: 10.1111/j.1442-9071.2009.02155.x. [DOI] [PubMed] [Google Scholar]
- 11.Oliveira M, Ribeiro H, Delgado JL, Abreu I. The effects of meteorological factors on airborne fungal spore concentration in two areas differing in urbanisation level. Int J Biometeorol. 2009;53:61–73. doi: 10.1007/s00484-008-0191-2. [DOI] [PubMed] [Google Scholar]
- 12.Siddiqui S, Anderson VL, Hilligoss DM, et al. Fulminant mulch pneumonitis: an emergency presentation of chronic granulomatous disease. Clin Infect Dis. 2007;45:673–81. doi: 10.1086/520985. [DOI] [PubMed] [Google Scholar]
- 13.Martire B, Rondelli R, Soresina A, et al. Clinical features, long-term follow-up and outcome of a large cohort of patients with chronic granulomatous disease: an Italian multicenter study. Clin Immunol. 2008;126:155–64. doi: 10.1016/j.clim.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 14.Gallien S, Fournier S, Porcher R, et al. Therapeutic outcome and prognostic factors of invasive aspergillosis in an infectious disease department: a review of 34 cases. Infection. 2008;36:533–8. doi: 10.1007/s15010-008-7375-x. [DOI] [PubMed] [Google Scholar]
- 15.Denning DW, Follansbee SE, Scolaro M, Norris S, Edelstein H, Stevens DA. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N Engl J Med. 1991;324:654–62. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 16.O’Donnell K, Sutton DA, Rinaldi MG, Gueidan C, Crous PW, Geiser DM. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum-F. equiseti and F. chlamydosporum species complexes within the United States. J Clin Microbiol. 2009;47:3851–61. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Donnell K, Sutton DA, Rinaldi MG, et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J Clin Microbiol. 2010;48:3708–18. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh S, Ramakrishnan R, Bharathi MJ, Amuthan M, Viswanathan S. Prevalence of bacterial pathogens causing ocular infections in South India. Indian J Pathol Microbiol. 2010;53:281–6. doi: 10.4103/0377-4929.64336. [DOI] [PubMed] [Google Scholar]
- 20.Hohl TM, Van Epps HL, Rivera A, et al. Aspergillus fumigatus triggers inflammatory responses by stage-specific beta-glucan display. PLoS Pathog. 2005;1:e30. doi: 10.1371/journal.ppat.0010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferwerda B, Ferwerda G, Plantinga TS, et al. Human dectin-1 deficiency and mucocutaneous fungal infections. N Engl J Med. 2009;361:1760–7. doi: 10.1056/NEJMoa0901053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Plantinga TS, van der Velden WJ, Ferwerda B, et al. Early stop polymorphism in human DECTIN-1 is associated with increased Candida colonization in hematopoietic stem cell transplant recipients. Clin Infect Dis. 2009;49:724–32. doi: 10.1086/604714. [DOI] [PubMed] [Google Scholar]
- 23.Glocker EO, Hennigs A, Nabavi M, et al. A homozygous CARD9 mutation in a family with susceptibility to fungal infections. N Engl J Med. 2009;361:1727–35. doi: 10.1056/NEJMoa0810719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bochud PY, Chien JW, Marr KA, et al. Toll-like receptor 4 polymorphisms and aspergillosis in stem-cell transplantation. N Engl J Med. 2008;359:1766–77. doi: 10.1056/NEJMoa0802629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leal SM, Jr, Cowden S, Hsia YC, Ghannoum MA, Momany M, Pearlman E. Distinct roles for Dectin-1 and TLR4 in the pathogenesis of Aspergillus fumigatus keratitis. PLoS Pathog. 2010;6:e1000976. doi: 10.1371/journal.ppat.1000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Werner JL, Metz AE, Horn D, et al. Requisite role for the dectin-1 beta-glucan receptor in pulmonary defense against Aspergillus fumigatus. J Immunol. 2009;182:4938–46. doi: 10.4049/jimmunol.0804250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Taylor PR, Tsoni SV, Willment JA, et al. Dectin-1 is required for beta-glucan recognition and control of fungal infection. Nat Immunol. 2007;8:31–8. doi: 10.1038/ni1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saijo S, Fujikado N, Furuta T, et al. Dectin-1 is required for host defense against Pneumocystis carinii but not against Candida albicans. Nat Immunol. 2007;8:39–46. doi: 10.1038/ni1425. [DOI] [PubMed] [Google Scholar]
- 29.McGreal EP, Rosas M, Brown GD, et al. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology. 2006;16:422–30. doi: 10.1093/glycob/cwj077. [DOI] [PubMed] [Google Scholar]
- 30.Robinson MJ, Osorio F, Rosas M, et al. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J Exp Med. 2009;206:2037–51. doi: 10.1084/jem.20082818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saijo S, Ikeda S, Yamabe K, et al. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity. 2010;32:681–91. doi: 10.1016/j.immuni.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Tarabishy AB, Aldabagh B, Sun Y, et al. MyD88 regulation of Fusarium keratitis is dependent on TLR4 and IL-1R1 but not TLR2. J Immunol. 2008;181:593–600. doi: 10.4049/jimmunol.181.1.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Netea MG, Gow NA, Munro CA, et al. Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. J Clin Invest. 2006;116:1642–50. doi: 10.1172/JCI27114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gross O, Poeck H, Bscheider M, et al. Syk kinase signalling couples to the Nlrp3 inflammasome for anti-fungal host defense. Nature. 2009;459:433–6. doi: 10.1038/nature07965. [DOI] [PubMed] [Google Scholar]
- 35.Hise AG, Tomalka J, Ganesan S, et al. An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe. 2009;5:487–97. doi: 10.1016/j.chom.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joly S, Ma N, Sadler JJ, Soll DR, Cassel SL, Sutterwala FS. Cutting edge: Candida albicans hyphae formation triggers activation of the Nlrp3 inflammasome. J Immunol. 2009;183:3578–81. doi: 10.4049/jimmunol.0901323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bellocchio S, Moretti S, Perruccio K, et al. TLRs govern neutrophil activity in aspergillosis. J Immunol. 2004;173:7406–15. doi: 10.4049/jimmunol.173.12.7406. [DOI] [PubMed] [Google Scholar]
- 38.Yuan X, Wilhelmus KR. Toll-like receptors involved in the pathogenesis of experimental Candida albicans keratitis. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.09-4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ramaprakash H, Ito T, Standiford TJ, Kunkel SL, Hogaboam CM. Toll-like receptor 9 modulates immune responses to Aspergillus fumigatus conidia in immunodeficient and allergic mice. Infect Immun. 2009;77:108–19. doi: 10.1128/IAI.00998-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasperkovitz PV, Cardenas ML, Vyas JM. TLR9 is actively recruited to Aspergillus fumigatus phagosomes and requires the N-terminal proteolytic cleavage domain for proper intracellular trafficking. J Immunol. 2010;185:7614–22. doi: 10.4049/jimmunol.1002760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y, Hise AG, Kalsow CM, Pearlman E. Staphylococcus aureus-induced corneal inflammation is dependent on Toll-like receptor 2 and myeloid differentiation factor 88. Infect Immun. 2006;74:5325–32. doi: 10.1128/IAI.00645-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun Y, Karmakar M, Roy S, et al. TLR4 and TLR5 on corneal macrophages regulate Pseudomonas aeruginosa keratitis by signaling through MyD88-dependent and -independent pathways. J Immunol. 2010;185:4272–83. doi: 10.4049/jimmunol.1000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hohl TM, Rivera A, Lipuma L, et al. Inflammatory monocytes facilitate adaptive CD4 T cell responses during respiratory fungal infection. Cell Host Microbe. 2009;6:470–81. doi: 10.1016/j.chom.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hebart H, Bollinger C, Fisch P, et al. Analysis of T-cell responses to Aspergillus fumigatus antigens in healthy individuals and patients with hematologic malignancies. Blood. 2002;100:4521–8. doi: 10.1182/blood-2002-01-0265. [DOI] [PubMed] [Google Scholar]
- 45.Hohl TM, Feldmesser M. Aspergillus fumigatus: principles of pathogenesis and host defense. Eukaryot Cell. 2007;6:1953–63. doi: 10.1128/EC.00274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.