Sir,
In comparison to modern medicine, the incidence of side-effects is lesser with herbal products, but they cannot be considered completely free from side-effects. The growing number of herbal drug users around the globe and scarcity of scientific reports regarding the safety aspects of herbal products make it imperative to conduct a toxicity study of herbal drugs.[1] Fumaria indica (FI), known as fumitory, is one of the commonly used medicinal plants in India.
Fumaria indica Linn. (Syn: Fumaria parviflora, Fumariaceae) is an herbaceous annual plant. The plant has been mentioned in Ayurveda, the traditional medicinal system of India as Pitapapra and claimed to possess various curative properties for ailments of the blood, skin, gastrointestinal system and central nervous system.[2] Recently, we have reported Fumaria indica as safe during an acute and subchronic oral toxicity study in rodents[3] and it displays central nervous system depressant activity.[4] So far there is no scientific report available regarding the chronic toxicity and cytotoxicity profile of Fumaria indica. Therefore, we decided to carry out the chronic toxicity and cytotoxicity study of a standardized extract of Fumaria indica.
Forty-eight adult Charles Foster albino rats (150±10 g) of either sex were distributed into three groups (T1, T2 and control). The rats were housed in polypropylene cages at an ambient temperature of 25°C±1°C and 45-55% relative humidity, with a 12:12 h light/dark cycle. Animals were provided with commercial food pellets and water ad libitum unless stated otherwise. The prior approval of the Institutional Animal Ethics Committee (IAEC) of Banaras Hindu University was obtained. Animals were acclimatized for at least one week before using them for the experiments. Principles of laboratory animal care (NIH publication number # 85-23, revised in 1985) guidelines were followed.
The plant Fumaria indica was collected from the local commercial source of Varanasi and identified by Prof. N. K. Dubey, Department of Botany, Faculty of Science, Banaras Hindu University. Herbarium specimen (specimen voucher, JAN- 2009- 01) of the plant was preserved. After shade drying, extraction of the plant was done with soxhlet apparatus using 50% ethanol as solvent.[5] Fifty percent ethanolic extract of Fumaria indica was standardized chemically by High-Performance Thin Layer Chromatography (HPTLC) through CAMAG TLC Scanner -III, Camag Linomat applicator IV using fumaric acid and di-methyl fumarate (Sigma-Aldrich, USA) as authentic markers.
FI extract was administered orally for 180 consecutive days[6] in doses of 100 and 400 mg/kg. Body weight, food and water consumption were monitored daily throughout the period of study. Control rats were given 0.3% carboxymethyl cellulose (CMC) suspension. For hematological and biochemical estimation blood was collected on Day 181 by retro-orbital technique. The kidney and liver of rats were isolated after sacrificing the rats to observe histopathological changes if any. Human monocytic cell line THP-1, at a density of 5 × 104 cells/well in a 96-well plate were used to assess the effect of FI on cell viability at concentration of 50 and 100 μg/ml. The viability of Tamm-Horsfall Protein-1 (THP-1) cells was measured in terms of mitochondrial metabolic activity using a colorimetric 4-[3-(4-iodophenyl)-2-(4-nitrophenyl)-2H-5-tetrazolio]-1,3-benzene disulfonate (water-soluble tetrazdium salt-1) - based cell proliferation assay (Millipore, MA, USA) according to the manufacturers’ instructions. Briefly, after 24 h treatment WST-1 was added to the cells and incubated for 4 h under standard culture conditions. Absorbance was measured at 450 nm using a microplate reader (Biorad, CA, USA). The results are expressed as Mean±SD. The data obtained from each response measure were subjected to Kruskal-Wallis one-way analysis of variance (ANOVA) and inter-group comparisons were made by Mann-Whitney U-test (two-tailed) for only those responses which yielded significant treatment effects in the ANOVA test.
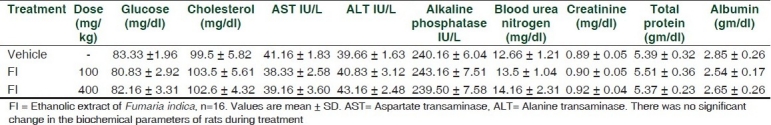
The test sample was found to contain 0.45% w/w of free fumaric acid and 0.35% w/w of fumaric acid conjugates (di-methyl fumarate). The body weight, daily food and water intake of FI-treated rats showed normal growth during six months of drug administration in comparison to vehicle-treated rats. Hematological findings revealed normal level of hemoglobin and total leukocyte count (TLC). Differential leukocyte count (DLC) was also found normal. Results are summarized in Table 1. The levels of glucose, cholesterol, alkaline phosphatase, aspartate transaminase (AST), alanine aminotransferase (ALT) , blood urea nitrogen, creatinine, total protein and albumin were not altered significantly in FI-treated rats compared to control rats. Results are summarized in Table 2. Histopathological examination of control and FI-treated rats revealed the absence of any gross pathological lesion in the kidney and liver. Examination of section of kidney in FI-treated rats showed normal renal architecture. Renal glomeruli, collecting tubules, interstitial tissue and blood vascular channels were found to be in normal condition. Interstitium of kidney was devoid of any kind of necrosis or degeneration. In the liver of FI-treated rats no focal or diffuse foci of necrosis of hepatocytes or infiltration of chronic inflammatory cells were observed. FI-treated rats showed normal lobular architecture of liver with normal central portal vein, radiating plates of hepatocytes and peripheral portal tracts composed of hepatic artery, bile ductule and distal portal vein. Cell line study using human monocytic cell line THP-1 indicated that FI did not have any toxic effect on the cells, rather it significantly promoted the growth of cells after 24 h of treatment. Overall findings of the study indicate for the first time that Fumaria indica is safe and devoid of toxic manifestations during chronic administration.
Table 1.
Effect of Fumaria indica on the hematological parameters of rats
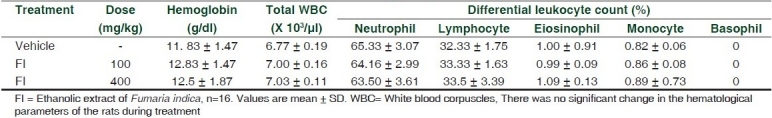
Table 2.
Effect of Fumaria indica on the biochemical parameters of rats
Acknowledgments
This study was supported by research funding from the Indian Council of Medical Research (ICMR), Government of India, New Delhi. Authors are also thankful to the R and D Centre, Indian Herbs Research and Supply Co. Ltd., Saharanpur, for carrying out the standardization of the plant extract used in this study.
REFERENCES
- 1.Saad B, Azaizeh H, Abu-Hijleh G, Said O. Safety of Traditional Arab Herbal Medicine. Evid Based Complement Alternat Med. 2006;3:433–9. doi: 10.1093/ecam/nel058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Usmanghani K, Saeed A, Alam MT. Karachi: University of Karachi Press; 1997. Indusyunic Medicine; pp. 240–1. [Google Scholar]
- 3.Singh GK, Kumar V. Acute and sub-chronic toxicity study of standardized extract of Fumaria indica in rodents. J Ethnopharmacol. 2011;134:992–5. doi: 10.1016/j.jep.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 4.Singh GK, Kumar V. Neuropharmacological screening and lack of antidepressant activity of standardized extract of Fumaria indica: A preclinical study. E J Pharmacol Therapy. 2010;3:19–28. [Google Scholar]
- 5.Rathi A, Srivastava AK, Shirwaikar A, Singh AK, Mehrotra S. Hepatoprotective potential of Fumaria indica Pugsley whole plant extracts, fractions and an isolated alkaloid protopine. Phytomedicine. 2008;15:470–7. doi: 10.1016/j.phymed.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Shin JW, Kim HG, Park HJ, Sung NW, Son CG. Safety of the traditional Korean herbal medicine CGX: A 6-month repeated-dose study in rats. J Ethnopharmacol. 2010;128:221–9. doi: 10.1016/j.jep.2010.01.019. [DOI] [PubMed] [Google Scholar]


