Abstract
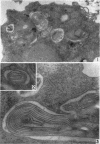
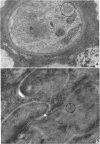
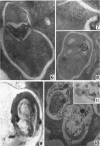
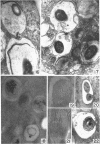
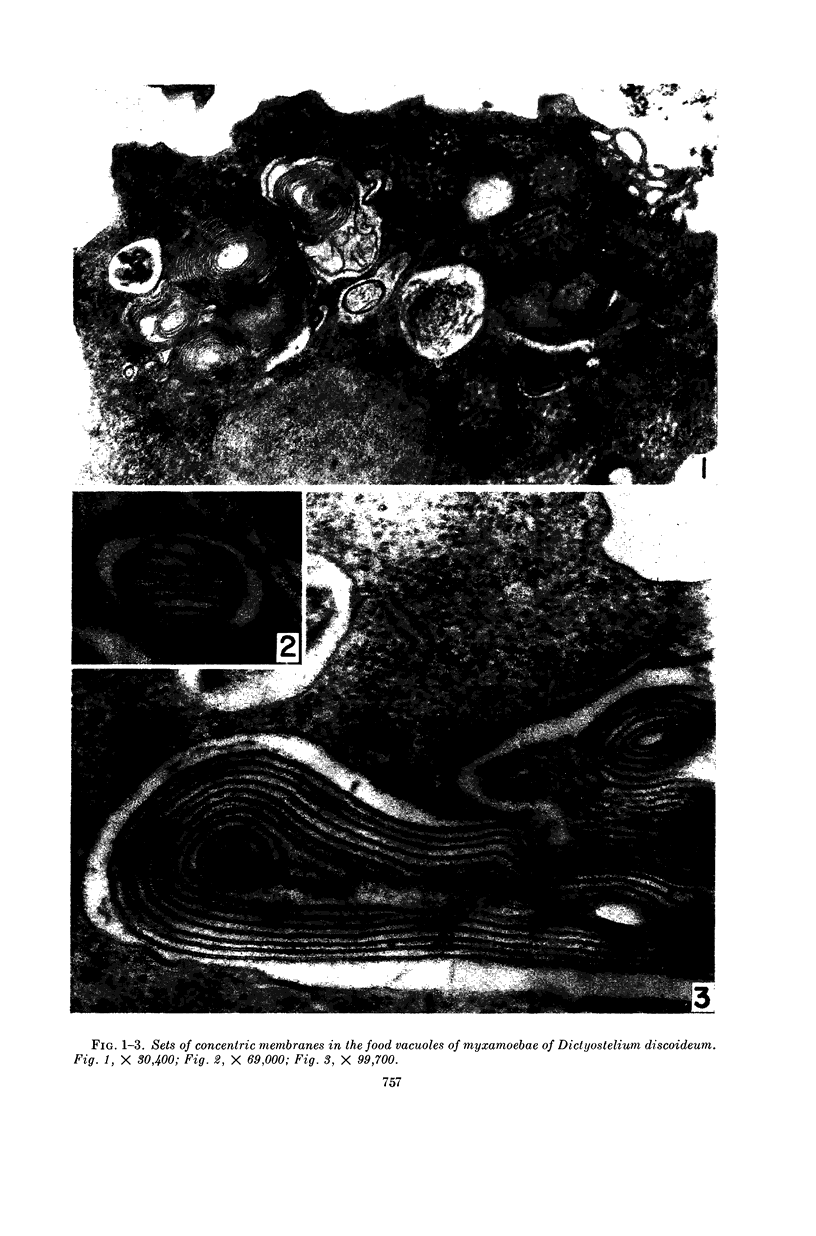
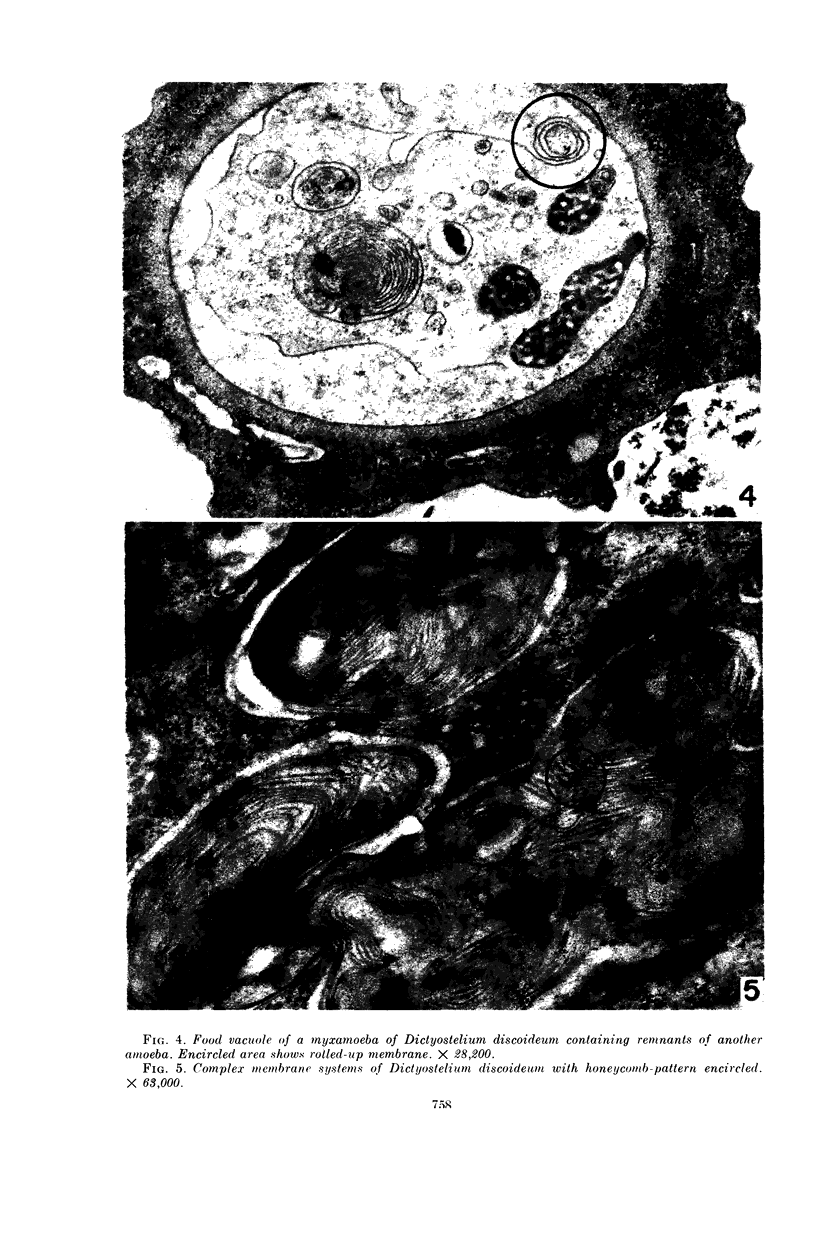
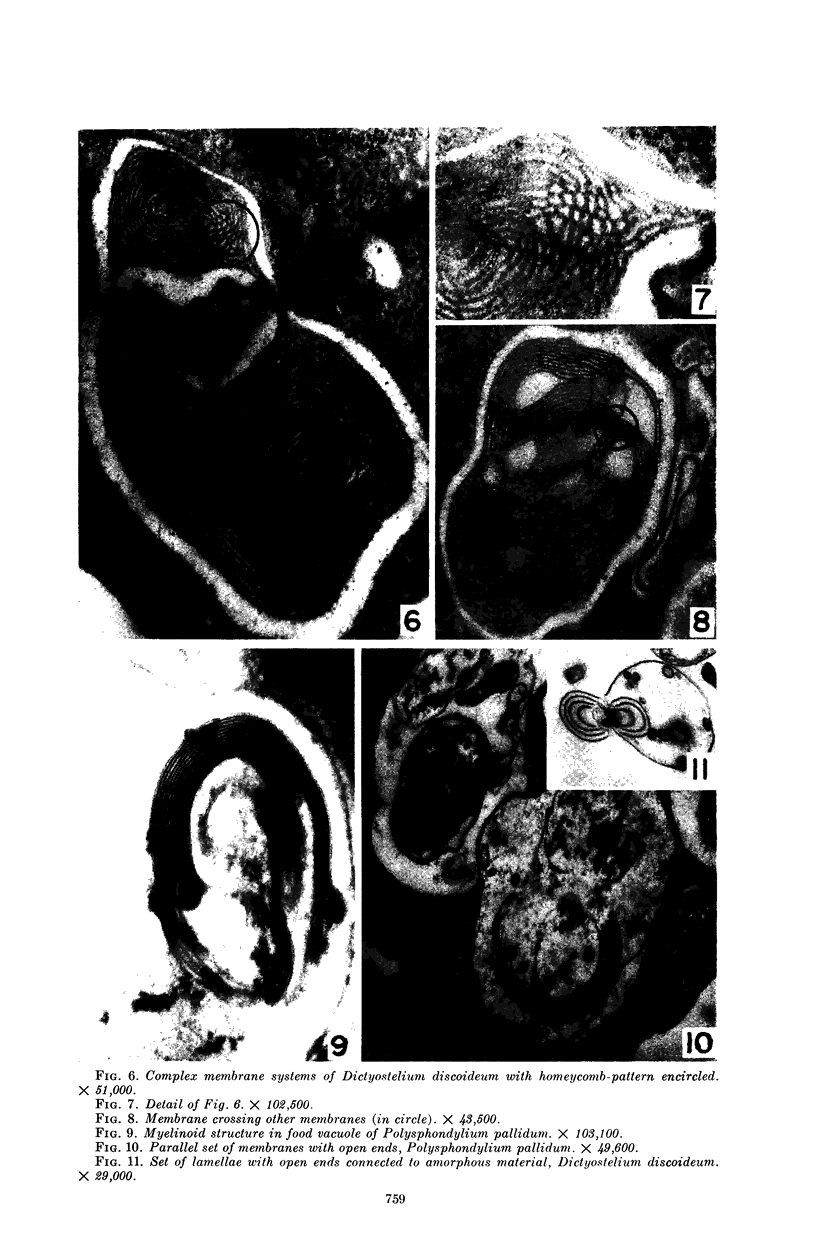
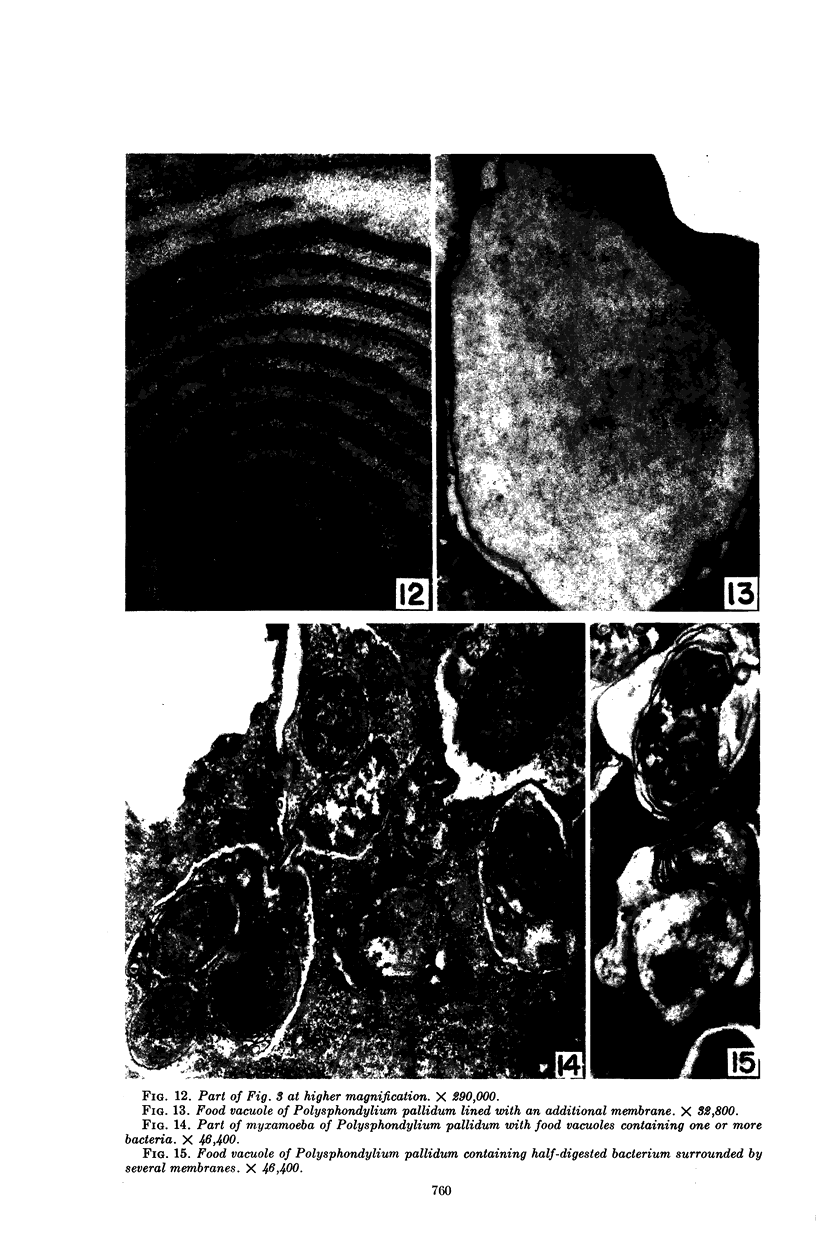
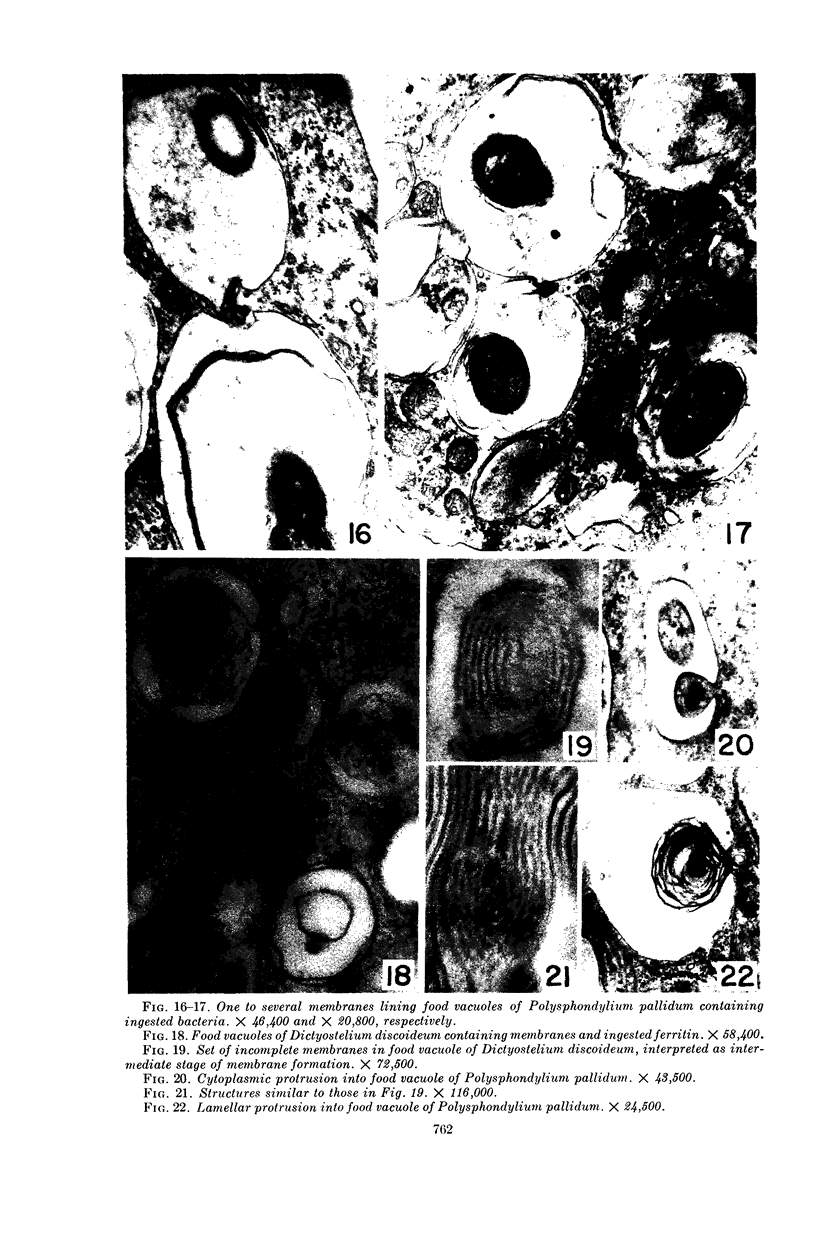
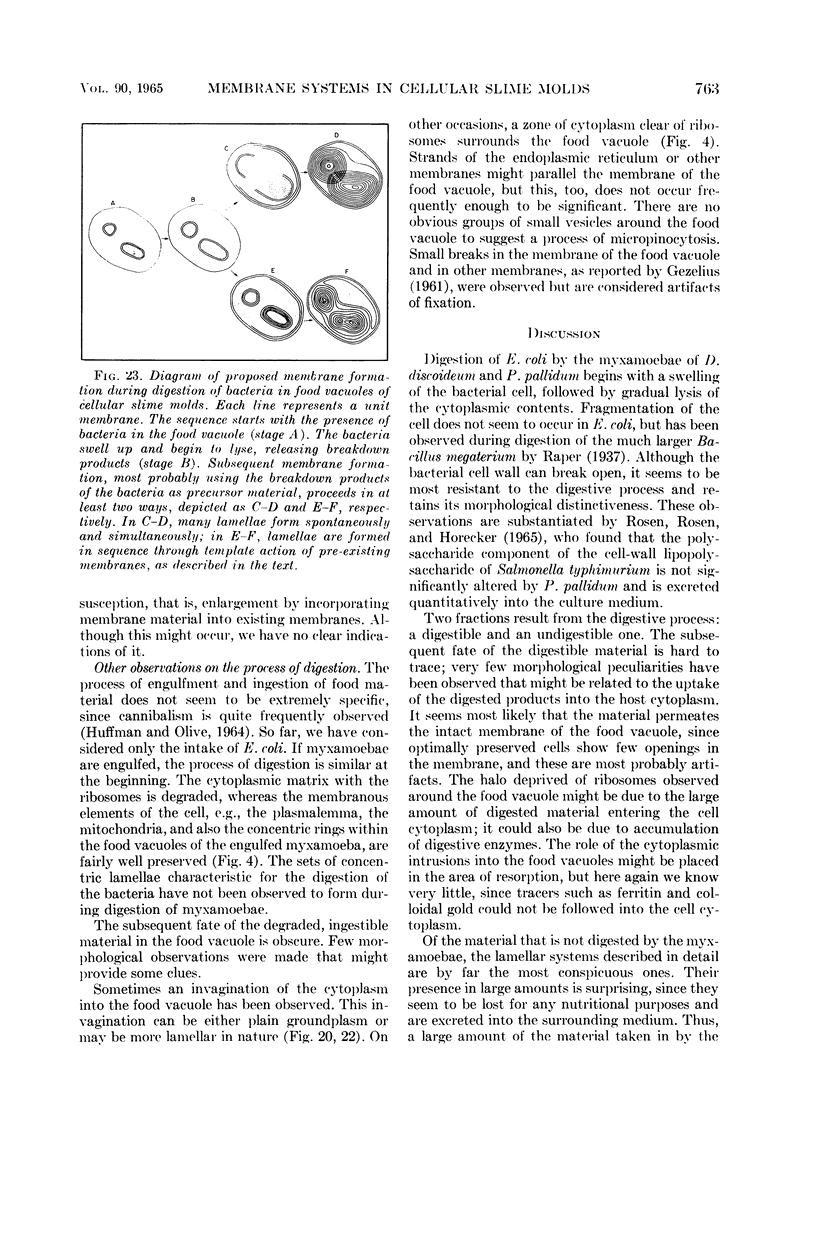
Hohl, Hans R. (University of Hawaii, Honolulu). Nature and development of membrane systems in food vacuoles of cellular slime molds predatory upon bacteria. J. Bacteriol. 90:755–765. 1965.—During the digestion of bacteria by the myxamoebae of cellular slime molds, systems of concentric lamellae begin to appear within the food vacuoles. Each constituent lamella is a unit membrane of 75 to 85 A thickness. A study of these lamellae in Dictyostelium discoideum and Polysphondylium pallidum reveals that most of them do not represent original membranes of the ingested bacteria but are formed mainly in two ways. (i) After swelling and partial digestion of the bacteria, the first membranes appear adjacent to pre-existing membranes, e.g., the membrane lining the food vacuole and the cytoplasmic membrane surrounding the bacterium. Progressive addition of lamellae leads to the formation of the systems of concentric lamellae. (ii) After digestion of the bacteria has proceeded to a high degree, the concentric lamellae are formed spontaneously from clouds of amorphous material through condensation and orientation of precursor material. The study shows that, in biological systems, unit membranes may be formed from amorphous material through template action of pre-existing membranes, and does not necessarily involve fusion of membrane-bound vesicles.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLONDEL B., TURIAN G. Relation between basophilia and fine structure of cytoplasm in the fungus Allomyces macrogynus Em. J Biophys Biochem Cytol. 1960 Feb;7:127–134. doi: 10.1083/jcb.7.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEZELIUS K. The ultrastructure of cells and cellulose membranes in Acrasiae. Exp Cell Res. 1959 Nov;18:425–453. doi: 10.1016/0014-4827(59)90310-6. [DOI] [PubMed] [Google Scholar]
- HOHL H. R., RAPER K. B. Nutrition of cellular slime molds. I. Growth on living and dead bacteria. J Bacteriol. 1963 Jan;85:191–198. doi: 10.1128/jb.85.1.191-198.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHL H. R., RAPER K. B. Nutrition of cellular slime molds. II. Growth of Polysphondylium pallidum in axenic culture. J Bacteriol. 1963 Jan;85:199–206. doi: 10.1128/jb.85.1.199-206.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOHL H., RAPER K. B. NUTRITION OF CELLULAR SLIME MOLDS. III. SPECIFIC GROWTH REQUIREMENTS OF POLYSPHONDYLIUM PALLIDUM. J Bacteriol. 1963 Dec;86:1314–1320. doi: 10.1128/jb.86.6.1314-1320.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUMPHREYS W. J. ELECTRON MICROSCOPE STUDIES OF THE FERTILIZED EGG AND THE TWO-CELL STAGE OF MYTILUS EDULIS. J Ultrastruct Res. 1964 Apr;10:244–262. doi: 10.1016/s0022-5320(64)80008-3. [DOI] [PubMed] [Google Scholar]
- KESSEL R. G. ELECTRON MICROSCOPE STUDIES ON THE ORIGIN OF ANNULATE LAMELLAE IN OOCYTES OF NECTURUS. J Cell Biol. 1963 Nov;19:391–414. doi: 10.1083/jcb.19.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUZZATI V., HUSSON F. The structure of the liquid-crystalline phasis of lipid-water systems. J Cell Biol. 1962 Feb;12:207–219. doi: 10.1083/jcb.12.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORRE D. J., MOLLENHAUER H. H. ISOLATION OF THE GOLGI APPARATUS FROM PLANT CELLS. J Cell Biol. 1964 Nov;23:295–305. doi: 10.1083/jcb.23.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OSEN O. M., ROSEN S. M., HORECKER B. L. FATE OF THE CELL WALL OF SALMONELLA TYPHIMURIUM UPON INGESTION BY THE CELLULAR SLIME MOLD: POLYSPHONDYLIUM PALLIDUM. Biochem Biophys Res Commun. 1965 Jan 18;18:270–276. doi: 10.1016/0006-291x(65)90752-7. [DOI] [PubMed] [Google Scholar]
- REBHUN L. I. Electron microscopy of basophilic structures of some invertebrate oocytes. II. Fine structure of the yolk nuclei. J Biophys Biochem Cytol. 1956 Mar 25;2(2):159–170. doi: 10.1083/jcb.2.2.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SLAUTTERBACK D. B. MITOCHONDRIA IN CARDIAC MUSCLE CELLS OF THE CANARY AND SOME OTHER BIRDS. J Cell Biol. 1965 Jan;24:1–21. doi: 10.1083/jcb.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUSSMAN M. Growth of the cellular slime mold Polysphondylium pallidum in a simple nutrient medium. Science. 1963 Jan 25;139(3552):338–338. doi: 10.1126/science.139.3552.338. [DOI] [PubMed] [Google Scholar]
- SWIFT H., HRUBAN Z. FOCAL DEGRADATION AS A BIOLOGICAL PROCESS. Fed Proc. 1964 Sep-Oct;23:1026–1037. [PubMed] [Google Scholar]
- SWIFT H. The fine structure of annulate lamellae. J Biophys Biochem Cytol. 1956 Jul 25;2(4 Suppl):415–418. doi: 10.1083/jcb.2.4.415. [DOI] [PMC free article] [PubMed] [Google Scholar]